Abstract
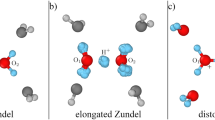
Compared to other ions, protons (H+) and hydroxide ions (OH-) exhibit anomalously high mobilities in aqueous solutions1. On a qualitative level, this behaviour has long been explained by ‘structural diffusion’—the continuous interconversion between hydration complexes driven by fluctuations in the solvation shell of the hydrated ions. Detailed investigations have led to a clear understanding of the proton transport mechanism at the molecular level2,3,4,5,6,7,8. In contrast, hydroxide ion mobility in basic solutions has received far less attention2,3,9,10, even though bases and base catalysis play important roles in many organic and biochemical reactions and in the chemical industry. The reason for this may be attributed to the century-old notion11 that a hydrated OH- can be regarded as a water molecule missing a proton, and that the transport mechanism of such a ‘proton hole’ can be inferred from that of an excess proton by simply reversing hydrogen bond polarities11,12,13,14,15,16,17,18. However, recent studies2,3 have identified OH- hydration complexes that bear little structural similarity to proton hydration complexes. Here we report the solution structures and transport mechanisms of hydrated hydroxide, which we obtained from first-principles computer simulations that explicitly treat quantum and thermal fluctuations of all nuclei19,20,21. We find that the transport mechanism, which differs significantly from the proton hole picture, involves an interplay between the previously identified hydration complexes2,3 and is strongly influenced by nuclear quantum effects.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Atkins, P. W. Physical Chemistry 6th edn 740–741 (Oxford Univ. Press, Oxford, 1998)
Tuckerman, M. E., Laasonen, K., Sprik, M. & Parrinello, M. Ab initio molecular dynamics simulation of the solvation and transport of H3O+ and OH- ions in water. J. Phys. Chem. 99, 5749–5752 (1995)
Tuckerman, M. E., Laasonen, K., Sprik, M. & Parrinello, M. Ab initio molecular dynamics simulation of the solvation and transport of hydronium and hydroxide ions in water. J. Chem. Phys. 103, 150–161 (1995)
Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 244, 456–462 (1995)
Marx, D., Tuckerman, M. E., Hutter, J. & Parrinello, M. The nature of the hydrated excess proton in water. Nature 397, 601–604 (1999)
Marx, D., Tuckerman, M. E. & Parrinello, M. Solvated excess protons in water: quantum effects on the hydration structure. J. Phys. Condens. Matter 12, A153–A159 (2000)
Vuilleumier, R. & Borgis, D. Transport and spectroscopy of the hydrated proton: A molecular dynamics study. J. Chem. Phys. 111, 4251–4266 (1999)
Day, T. J. F., Schmitt, U. W. & Voth, G. A. The mechanism of hydrated proton transport in water. J. Am. Chem. Soc. 122, 12027–12028 (2000)
Tuñón, I., Rinaldi, D., Ruiz-López, M. F. & Rivail, J. L. Hydroxide ion in liquid water: Structure, energetics, and proton transfer using a mixed discrete-continuum ab initio model. J. Phys. Chem. 99, 3798–3805 (1995)
Muller, R. P. & Warshel, A. Ab initio calculations of free energy barriers for chemical reactions in solution. J. Phys. Chem. 99, 17516–17524 (1995)
Hückel, E. Theorie der Beweglichkeiten des Wasserstoff- und Hydroxylions in wässriger Lösung. Z. Elektrochem. 34, 546–562 (1928)
Eigen, M. Proton transfer, acid-base catalysis, and enzymatic hydrolysis. Part I: Elementary processes. Angew. Chem. Int. Edn 3, 1–19 (1964)
Stillinger, F. H. in Theoretical Chemistry: Advances and Perspectives 177–234 (eds Eyring, H. & Henderson, D.) 177–234 (Academic, New York, 1978)
Zatsepina, G. N. State of the hydroxide ion in water and in aqueous solution. J. Struct. Chem. 12, 894–898 (1971)
Schiöberg, D. & Zundel, G. Very polarizable hydrogen bonds in solution of bases having infra-red absorption continua. J. Chem. Soc. Faraday Trans. II 69, 771–781 (1973)
Librovich, N. B., Sakun, V. P. & Sokolov, N. D. H+ and OH- ions in aqueous solutions—Vibrational spectra of hydrates. Chem. Phys. 39, 351–366 (1979)
Khoshtariya, D. E. & Berdzenishvili, N. O. A new dynamic elementary act model for thermal and photoinduced proton self-exchange through the lyate ion hydrogen bridges in solutions. Chem. Phys. Lett. 196, 607–613 (1992)
Agmon, N. Mechanism of hydroxide mobility. Chem. Phys. Lett. 319, 247–252 (2000)
Marx, D. & Parrinello, M. Ab initio path-integral molecular dynamics. Z. Phys. B 95, 143–144 (1994)
Tuckerman, M. E., Marx, D., Klein, M. L. & Parrinello, M. Efficient and general algorithms for path integral Car-Parrinello molecular dynamics. J. Chem. Phys. 104, 5579–5588 (1996)
Marx, D. & Hutter, J. in Modern Methods and Algorithms of Quantum Chemistry (ed. Grotendorst, J.) 301–449 (John von Neumann Institute for Computing, Forschungszentrum Jülich, 2000); also at 〈http://www.theochem.ruhr-uni-bochum.de/go/cprev.html〉 (2000)
Car, R. & Parrinello, M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett. 55, 2471–2474 (1985)
Novoa, J. J., Mota, F., del Valle, C. P. & Planas, M. Structure of the first solvation shell of the hydroxide anion. A model study using OH-(H2O)n (n = 4, 5, 6, 11, 17) clusters. J. Phys. Chem. A 101, 7842–7853 (1997)
Chaudhuri, C. et al. Infrared spectra and isomeric structures of hydroxide ion-water clusters OH-(H2O)1–5: a comparison with H3O+(H2O)1–5 . Mol. Phys. 99, 1161–1173 (2001)
Bruni, F., Ricci, M. A. & Soper, A. K. Structural characterization of NaOH aqueous solution in the glass and liquid states. J. Chem. Phys. 114, 8056–8063 (2001)
Buchner, R., Hefter, G., May, P. M. & Sipos, P. Dielectric relaxation of dilute aqueous NaOH, NaAl(OH)4 and NaB(OH)4 . J. Phys. Chem. B 103, 11186–11190 (1999)
Truhlar, D. G. & Kupperman, A. Exact tunneling calculations. J. Am. Chem. Soc. 93, 1840–1851 (1971)
Tuckerman, M. E., Marx, D., Klein, M. L. & Parrinello, M. On the quantum nature of the shared proton in hydrogen bonds. Science 275, 817–820 (1997)
Becke, A. D. & Edgecombe, K. E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 92, 5397–5403 (1990)
Trout, B. L. & Parrinello, M. Analysis of the dissociation of H2O in water using first-principles molecular dynamics. J. Phys. Chem. B 103, 7340–7345 (1999)
Liu, Y. & Tuckerman, M. E. Protonic defects in hydrogen bonded liquids: Structure and dynamics in ammonia and comparison with water. J. Phys. Chem. B 105, 6598–6610 (2001)
Geissler, P. L., Dellago, C., Chandler, D., Hutter, J. & Parrinello, M. Autoionization in liquid water. Science 291, 2121–2124 (2001)
Acknowledgements
M.E.T. was supported by the National Science Foundation (NSF) and Research Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Tuckerman, M., Marx, D. & Parrinello, M. The nature and transport mechanism of hydrated hydroxide ions in aqueous solution. Nature 417, 925–929 (2002). https://doi.org/10.1038/nature00797
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature00797
This article is cited by
-
Understanding hydrogen electrocatalysis by probing the hydrogen-bond network of water at the electrified Pt–solution interface
Nature Energy (2023)
-
Search for a Grotthuss mechanism through the observation of proton transfer
Communications Chemistry (2023)
-
Disentangling water, ion and polymer dynamics in an anion exchange membrane
Nature Materials (2022)
-
Synthesis, characterization, thermal properties and biological activity of diazine-ring containing hydrazones and their metal complexes
Journal of Thermal Analysis and Calorimetry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.