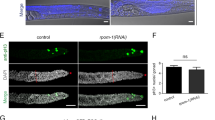
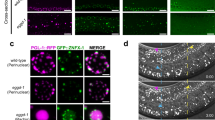
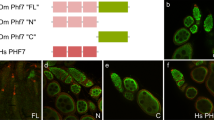
Abstract
Germline stem cells are defined by their unique ability to generate more of themselves as well as differentiated gametes1. The molecular mechanisms controlling the decision between self-renewal and differentiation are central unsolved problems in developmental biology with potentially broad medical implications. In Caenorhabditis elegans, germline stem cells are controlled by the somatic distal tip cell2,3. FBF-1 and FBF-2, two nearly identical proteins, which together are called FBF (‘fem-3 mRNA binding factor’), were originally discovered as regulators of germline sex determination4. Here we report that FBF also controls germline stem cells: in an fbf-1 fbf-2 double mutant, germline proliferation is initially normal, but stem cells are not maintained. We suggest that FBF controls germline stem cells, at least in part, by repressing gld-1, which itself promotes commitment to the meiotic cell cycle5,6. FBF belongs to the PUF family (‘Pumilio and FBF’) of RNA-binding proteins7. Pumilio controls germline stem cells in Drosophila females8,9, and, in lower eukaryotes, PUF proteins promote continued mitoses10,11. We suggest that regulation by PUF proteins may be an ancient and widespread mechanism for control of stem cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Watt, F. M. & Hogan, B. L. Out of Eden: stem cells and their niches. Science 287, 1427–1430 (2000)
Kimble, J. E. & White, J. G. On the control of germ cell development in Caenorhabditis elegans. Dev. Biol. 81, 208–219 (1981)
Schedl, T. in C. elegans II (ed. Priess, J. R.) 241–269 (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1997)
Zhang, B. et al. A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature 390, 477–484 (1997)
Francis, R., Barton, M. K., Kimble, J. & Schedl, T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics 139, 579–606 (1995)
Kadyk, L. C. & Kimble, J. Genetic regulation of entry into meiosis in Caenorhabditis elegans. Development 125, 1803–1813 (1998)
Wickens, M., Bernstein, D. S., Kimble, J. & Parker, R. A PUF family portrait: 3′UTR regulation as a way of life. Trends Genet. 18, 150–157 (2002)
Lin, H. & Spradling, A. C. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 124, 2463–2476 (1997)
Forbes, A. & Lehmann, R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 125, 679–690 (1998)
Souza, G. M., da Silva, A. M. & Kuspa, A. Starvation promotes Dictyostelium development by relieving PufA inhibition of PKA translation through the YakA kinase pathway. Development 126, 3263–3274 (1999)
Kennedy, B. K. et al. Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell 89, 381–391 (1997)
Dernburg, A. F. et al. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 94, 387–398 (1998)
Francis, R., Maine, E. & Schedl, T. Analysis of the multiple roles of gld-1 in germline development: interactions with the sex determination cascade and the glp-1 signalling pathway. Genetics 139, 607–630 (1995)
Jones, A. R., Francis, R. & Schedl, T. GLD-1, a cytoplasmic protein essential for oocyte differentiation, shows stage- and sex-specific expression during Caenorhabditis elegans germline development. Dev. Biol. 180, 165–183 (1996)
SenGupta, D. J. et al. A three-hybrid system to detect RNA-protein interactions in vivo. Proc. Natl Acad. Sci. USA 93, 8496–8501 (1996)
Bernstein, D. S., Buter, N., Stumpf, C. & Wickens, M. Analyzing mRNA–protein complexes using a yeast three-hybrid system: methods and applications. Methods 26(3), 123–141 (2002)
Jones, A. R. & Schedl, T. Mutations in gld-1, a female germ cell-specific tumor suppressor gene in Caenorhabditis elegans, affect a conserved domain also found in Src-associated protein Sam68. Genes Dev. 9, 1491–1504 (1995)
Jan, E., Motzny, C. K., Graves, L. E. & Goodwin, E. B. The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J. 18, 258–269 (1999)
Lee, M. H. & Schedl, T. Identification of in vivo mRNA targets of GLD-1, a maxi-KH motif containing protein required for C. elegans germ cell development. Genes Dev. 15, 2408–2420 (2001)
Xu, L., Paulsen, J., Yoo, Y., Goodwin, E. B. & Strome, S. Caenorhabditis elegans MES-3 is a target of GLD-1 and functions epigenetically in germline development. Genetics 159, 1007–1017 (2001)
Kraemer, B. et al. NANOS-3 and FBF proteins physically interact to control the sperm–oocyte switch in Caenorhabditis elegans. Curr. Biol. 9, 1009–1018 (1999)
Ward, S., Roberts, T. M., Strome, S., Pavalko, F. M. & Hogan, E. Monoclonal antibodies that recognize a polypeptide antigenic determinant shared by multiple Caenorhabditis elegans sperm-specific proteins. J. Cell Biol. 102, 1778–1786 (1986)
Crittenden, S. L., Troemel, E. R., Evans, T. C. & Kimble, J. GLP-1 is localized to the mitotic region of the C. elegans germ line. Development 120, 2901–2911 (1994)
Hendzel, M. J. et al. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma 106, 348–360 (1997)
Wharton, R. P. & Struhl, G. RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos. Cell 67, 955–967 (1991)
Tadauchi, T., Matsumoto, K., Herskowitz, I. & Irie, K. Post-transcriptional regulation through the HO 3′-UTR by Mpt5, a yeast homolog of Pumilio and FBF. EMBO J. 20, 552–561 (2001)
Acknowledgements
We thank R. Reijo Pera for sharing unpublished observations; and members of the Kimble and Wickens laboratories, T. Schedl and E. Goodwin for comments on the manuscript. J.K. is an investigator of the Howard Hughes Medical Institute. M.W. is supported by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Crittenden, S., Bernstein, D., Bachorik, J. et al. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature 417, 660–663 (2002). https://doi.org/10.1038/nature754
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature754
This article is cited by
-
Niche formation and function in developing tissue: studies from the Drosophila ovary
Cell Communication and Signaling (2023)
-
Mammalian pumilio proteins control cellular morphology, migration, and adhesion
Scientific Reports (2023)
-
Functional recovery of the germ line following splicing collapse
Cell Death & Differentiation (2022)
-
PUMILIO-mediated translational control of somatic cell cycle program promotes folliculogenesis and contributes to ovarian cancer progression
Cellular and Molecular Life Sciences (2022)
-
Transcriptional profiles in Strongyloides stercoralis males reveal deviations from the Caenorhabditis sex determination model
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.