Abstract
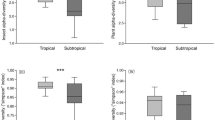
Patterns of association between herbivores and host plants have been thought to reflect the quality of plants as food resources1,2 as influenced by plant nutrient composition3, defences4,5, and phenology6. Host-plant-specific enemies, that is, the third trophic level, might also influence the distribution of herbivores across plant species7,8,9,10. However, studies of the evolution of herbivore host range11,12,13,14,15 have generally not examined the third trophic level, leaving unclear the importance of this factor in the evolution of plant–insect herbivore interactions16. Analysis of parasitoid rearings by the Canadian Forest Insect Survey shows that parasitism of particular Lepidoptera species is strongly host-plant-dependent, that the pattern of host-plant dependence varies among species of caterpillars, and that some parasitoid species are themselves specialized with respect to tree species. Host-plant-dependent parasitism suggests the possibility of top-down influence on host plant use. Differences in parasitism among particular caterpillar–host plant combinations could select for specialization of host plant ranges within caterpillar communities. Such specialization would ultimately promote the species diversification of Lepidoptera in temperate forests with respect to escape from enemies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ehrlich, P. R. & Raven, P. H. Butterflies and plants: a study of coevolution. Evolution 18, 586–608 (1964).
Cates, R G. Feeding patterns of monophagous, oligophagous, and polyphagous insect herbivores: the effect of resource abundance and plant chemistry. Oecologia 46, 22–31 (1980).
Scriber, J. M. & Feeny, P. Growth of herbivorous caterpillars in relation to feeding specialization and to the growth form of their food plants. Ecology 60, 829–850 (1979).
Courtney, S. P. Coevolution of pierid butterflies and their cruciferous host plants. III. Anthocaris cardamines (L.) survival, development, and oviposition on different host plants. Oecologia 51, 91–96 (1981).
Becerra, J. X. Insects on plants: macroevolutionary chemical trends in host use. Science 276, 253–256 (1997).
Wood, T. K. & Keese, M. C. Host-plant-induced assortative mating in Enchenopa tree hoppers. Evolution 44, 619–628 (1990).
Brower, L. P. Bird predation and food plant specificity in closely related procryptic insects. Am. Nat. 864, 886–892 (1958).
Hawkins, B. A. & Lawton, J. H. Species richness for parasitoids of British phytophagous insects. Nature 326, 788–790 (1987).
Bernays, E. & Graham, M. On the evolution of host specificity in phytophagous arthropods. Ecology 69, 886–892 (1988).
Holt, R. D. & Lawton, J. H. The ecological consequences of shared natural enemies. Annu. Rev. Ecol. Syst. 25, 495–520 (1994).
Feder, J. L., Chilcote, C. A. & Bush, G. L. Genetic differentiation between sympatric host races of the apple maggot fly Rhagoletis pomonella. Nature 336, 61–64 (1988).
Gould, F. Rapid host evolution in a population of the phytophagous mite Tetranychus urticae Koch. Evolution 33, 791–802 (1979).
Bush, G. L. Modes of animal speciation. Annu. Rev. Ecol. Syst. 6, 339–364 (1975).
Rausher, M. D. Variability for host preference in insect populations: mechanistic and evolutionary models. J. Insect Physiol. 31, 873–889 (1985).
Thompson, J. N. The Coevolutionary Process (Univ. Chicago Press, Chicago, 1994).
Jaenike, J. Host specialization in phytophagous insects. Annu. Rev. Ecol. Syst. 21, 243–273 (1990).
McGugan, B. M. The Canadian Forest Insect Survey. Proc. 10th Int. Congr. Entomol. 4, 219–232 (1958).
De Moraes, C. M., Lewis, W. J., Pare, P. W., Alborn, H. T. & Tumlinson, J. H. Herbivore-infested plants selectively attract parasitoids. Nature 393, 570–573 (1998).
Barbosa, P. & Benrey, B. in Conservation Biological Control (ed. Barbosa, P.) 55–82 (Academic, San Diego, 1998).
Le Corff, J., Marquis, R. J. & Whitfield, J. B. Temporal and spatial variation in a parasitoid community associated with the herbivores that feed on Missouri Quercus. Environ. Entomol. 29, 181–194 (2000).
Barbosa, P. et al. Differential parasitism of macrolepidopteran herbivores on two deciduous tree species. Ecology 82, 698–704 (2001).
Janzen, D. H. A host plant is more than it chemistry. Illinois Nat. Hist. Bull. 33, 141–174 (1985).
Elzen, G. W., Williams, H. J. & Vinson, S. B. Response by the parasitoid Campoletis sonorensis (Hyemonoptera: Ichneumonidae) to chemicals (synomones) in plants: implications for host habitat location. Environ. Entomol. 12, 1873–1877 (1983).
Weseloh, R. M. in Caterpillars: Ecological and evolutionary Constraints on Foraging (eds Stamp, N. E. & Casey, T. M.) 203–221 (Chapman & Hall, New York, 1993).
Greenblatt, J. A. & Barbosa, P. Effects of hosts' diet on two pupal parasitoids of the gypsy moth: Brachymeria intermedia (Nees) and Coccygomimus turionellae (L.). J. Appl. Ecol. 18, 1–19 (1981).
Mueller, T. F. The effect of plants on the host relations of a specialist parasitoid of Heliothis larvae. Ent. Exp. Appl. 34, 78–84 (1983).
Gross, P. & Price, P. W. Plant influences on parasitism of two leaf miners: a test of enemy-free space. Ecology 69, 1506–1516 (1988).
Ohsaki, N. & Sato, Y. Food plant choice of Pieris butterflies as a trade-off between parasitoid avoidance and quality of plants. Ecology 75, 59–68 (1994).
Karban, R. E. & Ricklefs, R. E. Host characteristics, sampling intensity, and species richness of Lepidoptera larvae on broad-leaved trees in southern Ontario. Ecology 64, 636–641 (1983).
Engels, W. Statistical Tests for Categorical Data Version 1.0 (software package) (Univ. Wisconsin, Madison, 1988).
Acknowledgements
We thank G. Howse for making available the CFIS data, E. Selig, A. Frevert, J. McGrath, and M. Walker for their efforts in constructing the database, B. Hawkins, K. Boege, G. Chen, R. Forkner, S. Renner, R. Rios and K. Schultz for their comments on the manuscript, and P. DeVries for software suggestions. Financial support for this project was provided by a University of Missouri Research Award to R.E.R. and R.J.M. Support for J.T.L. was provided by the Missouri Department of Conservation and the USDA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Lill, J., Marquis, R. & Ricklefs, R. Host plants influence parasitism of forest caterpillars. Nature 417, 170–173 (2002). https://doi.org/10.1038/417170a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/417170a
This article is cited by
-
Host plants of Phenacoccus solenopsis (Tinsley) affect parasitism of Aenasius bambawalei (Hayat)
Phytoparasitica (2022)
-
Tritrophic interaction diversity in gallery forests: A biologically rich and understudied component of the Brazilian cerrado
Arthropod-Plant Interactions (2021)
-
Host plant architecture affects the costs of dropping behaviour in Phaedon brassicae (Coleoptera: Chrysomelidae)
Applied Entomology and Zoology (2018)
-
Effects of herbivory and mistletoe infection by Psittacanthus calyculatus on nutritional quality and chemical defense of Quercus deserticola along Mexican forest fragments
Plant Ecology (2017)
-
Host use in Orgyia trigotephras (Erebidae, Lymantriinae) during outbreak: effects on larval performance and egg predation
Annals of Forest Science (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.