Abstract
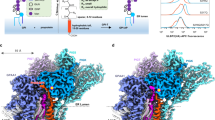
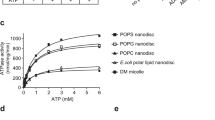
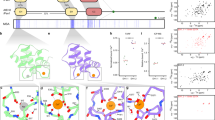
Specific sorting signals direct transmembrane proteins to the compartments of the endosomal–lysosomal system1. Acidic-cluster-dileucine signals present within the cytoplasmic tails of sorting receptors, such as the cation-independent and cation-dependent mannose-6-phosphate receptors, are recognized by the GGA (Golgi-localized, γ-ear-containing, ADP-ribosylation-factor-binding) proteins2,3,4,5. The VHS (Vps27p, Hrs and STAM) domains6 of the GGA proteins are responsible for the highly specific recognition of these acidic-cluster-dileucine signals7,8,9,10. Here we report the structures of the VHS domain of human GGA3 complexed with signals from both mannose-6-phosphate receptors. The signals bind in an extended conformation to helices 6 and 8 of the VHS domain. The structures highlight an Asp residue separated by two residues from a dileucine sequence as critical recognition elements. The side chains of the Asp-X-X-Leu-Leu sequence interact with subsites consisting of one electropositive and two shallow hydrophobic pockets, respectively. The rigid spatial alignment of the three binding subsites leads to high specificity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kirchhausen, T. Clathrin. Annu. Rev. Biochem. 69, 699–727 (2000).
Dell’Angelica, E. C. et al. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 149, 81–94 (2000).
Hirst, J. et al. A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol. 149, 67–80 (2000).
Boman, A. L., Zhang, C.-J., Zhu, X. & Kahn, R. A. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell 11, 1241–1255 (2000).
Poussu, A., Lohi, O. & Lehto, V.-P. Vear, a novel Golgi-associated protein with VHS and gamma-adaptin ‘ear’ domains. J. Biol. Chem. 275, 7176–7183 (2000).
Lohi, O. & Lehto, V.-P. VHS domain marks a group of proteins involved in endocytosis and vesicular trafficking. FEBS Lett. 440, 255–257 (1998).
Puertollano, R., Aguilar, R. C., Gorshkova, I., Crouch, R. J. & Bonifacino, J. S. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science 292, 1712–1716 (2001).
Zhu, Y., Doray, B., Poussu, A., Lehto, V.-P. & Kornfeld, S. Binding of GGA2 to the lysosomal enzyme sorting motif of the mannose 6-phosphate receptor. Science 292, 1716–1718 (2001).
Takatsu, H., Katoh, Y., Shiba, Y. & Nakayama, K. GGA proteins interact with acidic di-leucine sequences within the cytoplasmic domains of sorting receptors through their VHS domains. J. Biol. Chem. 276, 28541–28545 (2001).
Nielsen, M. S. et al. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 20, 2180–2190 (2001).
Mao, Y. et al. Crystal structure of the VHS and FYVE tandem domains of Hrs, a protein involved in membrane trafficking and signal transduction. Cell 100, 447–456 (2000).
Misra, S., Beach, B. M. & Hurley, J. H. Structure of the VHS domain of human Tom1 (target of myb 1): insights into interactions with proteins and membranes. Biochemistry 39, 11282–11290 (2000).
Chen, H. J., Yuan, J. & Lobel, P. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J. Biol. Chem. 272, 7003–7012 (1997).
Johnson, K. F. & Kornfeld, S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J. Cell. Biol. 119, 249–257 (1992).
Meresse, S. & Hoflack, B. J. Phosphorylation of the cation-independent mannose 6-phosphate receptor is closely associated with its exit from the trans-Golgi network. J. Cell Biol. 120, 67–75 (1993).
Owen, D. J. & Evans, P. R. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282, 1327–1332 (1998).
Gatto, G. J. Jr, Geisbrecht, B. V., Gould, S. J. & Berg, J. M. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nature Struct. Biol. 7, 1091–1095 (2000).
Scheufler, C. et al. Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70–Hsp90 multichaperone machine. Cell 101, 199–210 (2000).
Sheffield, P., Garrard, S. & Derewenda, Z. Overcoming expression and purification problems of RhoGDI using a family of ‘parallel’ expression vectors. Prot. Express. Purif. 15, 34–39 (1999).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Terwilliger, T. C. & Berendzen., J. Automated MAD and MIR structure solution. Acta Crystallogr. D 55, 849–861 (1999).
Terwilliger, T. C. Maximum-likelihood density modification. Acta Crystallogr. D 56, 965–972 (2000).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjelgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991).
Brünger, A. T. et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Esnouf, R. M. Further additions to MolScript version 1.4, including reading and contouring of electron-density maps. Acta Crystallogr. D 55, 938–940 (1999).
Merritt, E. A. & Bacon, D. J. Raster3D: photorealistic molecular graphics. Methods Enzymol. 277, 505–524 (1997).
Acknowledgements
We thank K. R. Rajashankar, U. Ramagopal, R. Trievel and E. Jones for assistance with data collection at beamline X9B, NSLS, D. E. Anderson for mass spectrometry, A. Peterkofsky for use of and assistance with the titration calorimeter, R. Ghirlando for analytical ultracentrifugation, X. Zhu for sequencing services, and H. Bernstein and P. Sun for comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Misra, S., Puertollano, R., Kato, Y. et al. Structural basis for acidic-cluster-dileucine sorting-signal recognition by VHS domains. Nature 415, 933–937 (2002). https://doi.org/10.1038/415933a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/415933a
This article is cited by
-
A Naturally Occurring Splice Variant of GGA1 Inhibits the Anterograde Post-Golgi Traffic of α2B-Adrenergic Receptor
Scientific Reports (2019)
-
Insulin-Like Growth Factor-II/Cation-Independent Mannose 6-Phosphate Receptor in Neurodegenerative Diseases
Molecular Neurobiology (2017)
-
A bacterial genetic selection system for ubiquitylation cascade discovery
Nature Methods (2016)
-
Regulation of α2B-Adrenergic Receptor Cell Surface Transport by GGA1 and GGA2
Scientific Reports (2016)
-
Coupling of lysosomal and mitochondrial membrane permeabilization in trypanolysis by APOL1
Nature Communications (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.