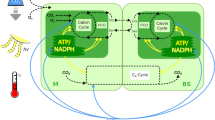
Abstract
Most plants are known as C3 plants because the first product of photosynthetic CO2 fixation is a three-carbon compound1. C4 plants, which use an alternative pathway in which the first product is a four-carbon compound, have evolved independently many times and are found in at least 18 families2,3. In addition to differences in their biochemistry, photosynthetic organs of C4 plants show alterations in their anatomy and ultrastructure4. Little is known about whether the biochemical or anatomical characteristics of C4 photosynthesis evolved first. Here we report that tobacco, a typical C3 plant, shows characteristics of C4 photosynthesis in cells of stems and petioles that surround the xylem and phloem, and that these cells are supplied with carbon for photosynthesis from the vascular system and not from stomata. These photosynthetic cells possess high activities of enzymes characteristic of C4 photosynthesis, which allow the decarboxylation of four-carbon organic acids from the xylem and phloem, thus releasing CO2 for photosynthesis. These biochemical characteristics of C4 photosynthesis in cells around the vascular bundles of stems of C3 plants might explain why C4 photosynthesis has evolved independently many times.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Calvin, M. et al. Carbon dioxide assimilation in plants. Symp. Soc. Exp. Biol. 5, 284–305 (1951).
Moore, P. D. Evolution of photosynthetic pathways in flowering plants. Nature 310, 696 (1982).
Sage, R. F., Li, M. & Monson, R. K. in C4 Plant Biology (eds Sage, R. F. & Monson, R. K.) 551–584 (Academic, San Diego, 1999).
Hatch, M. D. C4 photosynthesis; a unique blend of modified biochemistry, anatomy and ultrastructure. Biochem. Biophys. Acta 895, 81–106 (1987).
Reinfelder, J. R., Kraepiel, A. M. L. & Morel, F. M. Unicellular C4 photosynthesis in a marine diatom. Nature 407, 996–999 (2000).
Monson, R. K. in C4 Plant Biology (eds Sage R. F. & Monson, R. K.) 377–410 (Academic, San Diego, 1999).
Rawsthorne, S. C3–C4 intermediate photosynthesis: linking physiology to gene expression. Plant J. 2, 267–274 (1992).
Rawsthorne, S., Hylton, C. M., Smith, A. M. & Woolhouse, H. W. Photorespiratory and immunogold localization of photorespiratory enzymes in leaves of C3 and C3–C4 intermediate species of Moricandia. Planta 173, 298–308 (1988).
Rawsthorne, S., Hylton, C. M., Smith, A. M. & Woolhouse, H. W. Distribution of photorespiratory enzymes between bundle sheath and mesophyll cells in leaves of the C3–C4 intermediate species Moricandia arvensis (L.) DC. Planta 176, 527–532 (1988).
Keeley, J. E. CAM photosynthesis in submerged aquatic plants. Bot. Rev. 64, 121–175 (1998).
Keeley, J. E., Osmond, C. B. & Raven, J. A. Stylites, a vascular land plant without stomata absorbs CO2 via its roots. Nature 310, 694–695 (1984).
Hibberd, J. M. et al. Localization of photosynthetic metabolism in the parasitic angiosperm Cuscuta reflexa. Planta 205, 506–513 (1998).
Esau, K. Plant Anatomy 2nd edn (John Wiley, New York, 1965).
Kinsman, E. A. & Pyke, K. A. Bundle sheath cells and cell-specific plastid development in Arabidopsis leaves. Development 125, 1815–1822 (1998).
Nilsen, E. T. in Plant Stems (ed. Gartner, B. L.) 223–240 (Academic, San Diego, 1995).
Raven, J. A. Long-term functioning of enucleate sieve elements: possible mechanisms of damage avoidance and damage repair. Plant Cell Environ. 14, 139–146 (1991).
Gerendas, J. & Schurr, U. Physicochemical aspects of ion relations and pH regulation in plants—a quantitative approach. J. Exp. Bot. 50, 1101–1114 (1999).
Cramer, M. D. & Richards, M. B. The effect of rhizosphere dissolved inorganic carbon on gas exchange characteristics and growth rates of tomato seedlings. J. Exp. Bot. 50, 79–87 (1999).
Cramer, M. D., Gao, Z. F. & Lips, S. H. The influence of dissolved inorganic carbon in the rhizosphere on carbon and nitrogen metabolism in salinity treated tomato plants. New Phytol. 142, 441–450 (1999).
Vuorinen, A. R., Rossi, P. & Vapaavuori, E. M. Combined effect of inorganic carbon and different nitrogen sources in the growth media on biomass production and nitrogen uptake in young willow and birch plants. J. Plant Physiol. 147, 236–242 (1995).
Pate, J. S. in Encylopedia of Plant Physiology Vol. 1 (eds Zimmerman, M. H. & Milburn, J. A. ) 451–473 (Springer, Heidelberg, 1975).
Raven, J. A. & Smith, F. A. Nitrogen assimilation and transport in vascular land plants in relation to intracellular pH regulation. New Phytol. 76, 415–431 (1976).
Ben Zioni, A., Vaadia, Y. & Lips, S. H. Nitrate uptake by roots as regulated by nitrate reduction products of the shoot. Physiol. Plant. 24, 288–290 (1971).
Hibberd, J. M., Quick, W. P., Press, M. C., Scholes, J. D. & Jeschke, W. D. Solute fluxes from tobacco to the parasitic angiosperm Orobanche cernua and the influence of infection on host carbon and nitrogen relations. Plant Cell Environ. 22, 937–947 (1999).
Ashton, A. R., Burnell, J. N., Furbank, R. T., Jenkins, C. L. D. & Hatch, M. D. in Methods in Plant Biochemistry, Vol. 3 (ed. Lea, P. J.) 39–72 (Academic, San Diego, 1990).
Lea, P. J. & Leegood, R. C. Plant Biochemistry and Molecular Biology (Wiley, Chichester, 1999).
Schaaf, J., Walter, M. H. & Hess, D. Primary metabolism in plant defence. Plant Physiol. 108, 949–960 (1995).
Gao, Z., Sagi, M. & Lips, M. Assimilate allocation priority as affected by nitrogen compounds in the xylem sap of tomato. Plant Physiol. Biochem. 34, 807–815 (1996).
Thomas, J. C., De Armond, R. L. & Bohnert, H. J. Influence of NaCl on growth, proline and phosphoenolpyruvate carboxylase levels in Mesembryanthemum crystallinum suspension cultures. Plant Physiol. 98, 626–631 (1992).
Walker, R. P., Trevanion, S. J. & Leegood, R. C. Phosphenolpyruvate carboxykinase from higher plants: Purification from cucumber and evidence of rapid proteolytic cleavage in extracts from a range of plant tissues. Planta 196, 58–63 (1995).
Acknowledgements
We thank J. C. Gray and W. D. Jeschke for discussions. J.M.H. thanks the BBSRC for a Sir David Phillips Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Hibberd, J., Quick, W. Characteristics of C4 photosynthesis in stems and petioles of C3 flowering plants. Nature 415, 451–454 (2002). https://doi.org/10.1038/415451a
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/415451a
This article is cited by
-
Role of C4 photosynthetic enzyme isoforms in C3 plants and their potential applications in improving agronomic traits in crops
Photosynthesis Research (2022)
-
Photosynthetic contribution and characteristics of cucumber stems and petioles
BMC Plant Biology (2021)
-
A rotary mechanism for allostery in bacterial hybrid malic enzymes
Nature Communications (2021)
-
Manganese toxicity disrupts indole acetic acid homeostasis and suppresses the CO2 assimilation reaction in rice leaves
Scientific Reports (2021)
-
Physiological and transcriptomic responses of Mikania micrantha stem to shading yield novel insights into its invasiveness
Biological Invasions (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.