Abstract
The oceanic picoplankton Prochlorococcus — probably the most abundant photosynthetic organism on our planet1,2 — can grow at great depths where light intensity is very low3. We have found that the chlorophyll-binding proteins in a deep-living strain of this oxyphotobacterium form a ring around a trimer of the photosystem I (PS I) photosynthetic reaction centre, a clever arrangement that maximizes the capture of light energy in such dim conditions.
Similar content being viewed by others
Main
Many types of oxyphotobacterium collect light for photosynthesis by means of water-soluble phycobilisomes, but Prochlorococcus, Prochloron and Prochlorothrix all use intrinsic light-collecting proteins that bind to chlorophyll a or b. Consequently, it was thought that the chloroplasts of green algae and higher plants, which also contain chlorophyll a/b-binding proteins, were derived by endosymbiosis from an ancestral prokaryote phylogenetically related to one of these three atypical oxyphotobacteria4.
Gene sequencing has shown, however, that the pcb genes that encode the principal light-collecting proteins are not closely related to the cab genes that encode the chlorophyll a/b-binding proteins of green plastids5, but instead are similar to the iron-stress-induced isiA gene of cyanobacteria5. Both encode proteins predicted to have six transmembrane helices that share homology with the six transmembrane helices of CP43, a protein that binds to chlorophyll a in photosystem II (PS II).
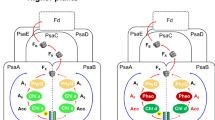
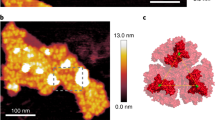
We investigated how the chlorophyll a/b-binding Pcb proteins structurally interact with the PS I reaction centre in Prochlorococcus. Figure 1a shows a projection map derived from electron microscopy and single-particle analysis of a large PS I complex isolated from Prochlorococcus strain SS120 by centrifugation through a density gradient of sucrose. The central region of the image has threefold symmetry and is surrounded by 18 separate subunits, as inferred from their densities.
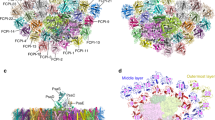
a, Projection of the top view of the Pcb–PS I supercomplex isolated from Prochlorococcus marinus, strain SS120. This map is derived from the initial analyses of 3,500 negatively stained particles viewed under electron microscopy at room temperature (scale bar, 5 nm). b, c, Top view from the stromal side (b), and side view showing the extrinsic domains on the stromal side (c) of a three-dimensional model of the Pcb–PS I supercomplex; the model is based on X-ray data from cyanobacterial PS I trimers10 (green) and CP43 transmembrane helices of cyanobacterial photosystem II (ref. 8; red).
This structure is remarkably similar to that of the PS I complex isolated from the cyanobacterium Synechocystis PCC6803 after iron deprivation6. In this case, the central region consists of a trimer of the PS I reaction centre surrounded by 18 copies of the isiA gene product. Given the sequence similarity between the isiA and pcb genes, the structure shown in Fig. 1a might also represent a trimer of PS I surrounded by 18 copies of one or more of the 7 chlorophyll a/b-binding Pcb proteins found in this Prochlorococcus strain7.
Chlorophyll fluorescence and protein analysis confirmed that this new complex contained PS I and Pcb proteins, and that efficient energy transfer occurs from the Pcb antenna ring to the core of the reaction centre. Furthermore, optical absorption spectra indicated that chlorophyll a and b are contained within the antenna ring and also within the PS I trimer. Figure 1b, c shows the three-dimensional structure of this Pcb–PS I supercomplex. This was modelled using the X-ray structures of the PS I trimer and CP43 transmem-brane helices8,9 of the cyanobacterium Synechococcus elongatus.
The Prochlorococcus strain used here was originally collected from the Sargasso Sea2,10, where it grows at considerable depths and would benefit from an antenna system of the type reported here in order to increase the collecting capacity of PS I. The ring of Pcb proteins contributes a further 270 light-collecting chlorophyll molecules to the 300 predicted to be associated with the PS I reaction-centre trimer, assuming that the Pcb subunits each bind to 15 chlorophyll molecules.
The antenna ring therefore represents an increase of 90% in light-collecting capacity by the Pcb–PS I supercomplex over that of PS I in cyanobacteria such as Synechocystis, when growing in the absence of iron stress. In this latter case, the cyanobacterial PS I/PS II ratio is about 3:1, compared with 1:1 in Prochlorococcus. Under iron-stress conditions, however, when the IsiA antenna ring forms around PS I, the PS I/PS II ratio drops to about 1: 1 in cyanobacteria as well. The formation of these Pcb and IsiA rings around PS I may therefore represent a mechanism for increasing the efficiency of this photosystem when its level is close to that of PS II.
It is not clear whether the PS I antenna ring is present in other types of chlorophyll b-containing oxyphotobacteria, as the number of pcb genes differs in Prochloron, Prochlorothrix and Prochlorococcus, as well as among different strains of Prochlorococcus5,7. Although the relationship between gene number and structural features in other strains of Prochlorococcus remains to be investigated, our discovery of an antenna ring around PS I in both Prochlorococcus and iron-deficient cyanobacteria6 opens up discussion on the regulation of light-collection systems in oxyphotobacteria, as well as having implications for the evolution of these photosynthetic prokaryotes. The structures give functional significance to the trimerization of PS I in oxyphotobacteria, as well as providing new systems for studying energy transfer in light-collecting antennae during photosynthesis.
References
Chisholm, S. W. et al. Nature 334, 340–343 (1988).
Chisholm, S. W. et al. Arch. Microbiol. 157, 297–300 (1992).
Partensky, F., Hess, W. R. & Vaulot, D. Microbiol. Mol. Biol. Rev. 63, 106–127 (1999).
Lewin, R. A. Nature 261, 697–698 (1976).
La Roche, J. et al. Proc. Natl Acad. Sci. USA 93, 15244–15248 (1996).
Bibby, T. S., Nield, J. & Barber, J. Nature 412, 743–745 (2001).
Garczarek, L. et al. Proc. Natl Acad. Sci. USA 97, 4098–4101 (2000).
Zouni, A. et al. Nature 409, 739–743 (2001).
Krauss, N. et al. Nature Struct. Biol. 3, 965–973 (1996).
Moore, L. R., Goericke, R. & Chisholm, S. W. Ecol. Prog. Ser. 116, 259–275 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bibby, T., Nield, J., Partensky, F. et al. Antenna ring around photosystem I. Nature 413, 590 (2001). https://doi.org/10.1038/35098153
Issue Date:
DOI: https://doi.org/10.1038/35098153
This article is cited by
-
Persistent equatorial Pacific iron limitation under ENSO forcing
Nature (2023)
-
Soil potassium is correlated with root secondary metabolites and root-associated core bacteria in licorice of different ages
Plant and Soil (2020)
-
Membrane organization of photosystem I complexes in the most abundant phototroph on Earth
Nature Plants (2019)
-
Chlorophyll d and Acaryochloris marina: current status
Photosynthesis Research (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.