Abstract
The ordered domain patterns that form spontaneously in a wide variety of chemical and physical systems1,2 as a result of competing interatomic interactions can be used as templates for fabricating nanostructures. Here we describe a new self-assembling domain pattern on a solid surface that involves two surface structures of lead on copper. The evolution of the system agrees with theoretical predictions3,4,5, enabling us to probe the interatomic force parameters that are crucial to the process.
Similar content being viewed by others
Main
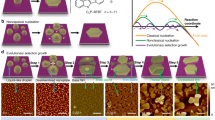
The two structures showing this remarkable behaviour are a disordered Pb/Cu surface alloy, produced by deposition of lead vapour onto clean Cu(111), and a lead-overlayer phase, which is produced by continued deposition after the alloy structure has covered the surface6,7,8. Figure 1a–g shows a sequence of low-energy electron microscope (LEEM) images in which the overlayer structure (bright) grows over the surface alloy structure (dark) during lead deposition at a temperature of 673 K. The most striking feature of this sequence is the evolution of a pattern from circular islands (average diameter, 67 nm) to stripes and then to circular holes within the lead-overlayer matrix.
Low-energy electron micrographs of the Cu(111) surface at 673 K with different area fractions of the lead-overlayer phase (bright) in the surface alloy phase (dark). a–g, Area fractions 0.03, 0.28, 0.35, 0.50, 0.65, 0.73 and 0.95, respectively. The domain pattern evolves from circular islands (droplets) to stripes, to vacancy islands (inverted droplets) with increasing lead coverage. h, Ordered droplet configuration at 623 K. Scale bar, 0.5 μm. i, Atomic-force micrograph of a droplet pattern after cooling down to room temperature and 2 hours of exposure to air. Scale bar, 0.3 μm.
This sequence of domain patterns, referred to as droplets, stripes and inverted droplets, respectively, has been predicted to be a general property of domain formation on solid surfaces3,4,5,9,10. To our knowledge, these results represent the first unambiguous observation of the expected sequence of domain patterns with changing area fraction during deposition, and agree with the predicted positions5 of the droplet-to-stripe phase transitions for short-range attractive and long-range dipolar interatomic interactions on solid surfaces.
One reason why these domain patterns form in this system is the high mobility of the islands (Fig. 1a), which contain many thousands of atoms and can move by as much as a micrometre before being incorporated into the more tightly packed droplet structure (Fig. 1b). This mobility allows patterns to form that have hundred-nanometre periodicity and allows the underlying interatomic interactions to be determined.
As the islands move in the force fields of the other islands, the island trajectories contain information about the force fields. Droplet trajectories are consistent with the expected long-range dipolar repulsion. Because magnetic interactions are not relevant in this system and we can rule out electrostatic interactions from measurements of work-function differences, we propose that elastic interactions arising from a surface-stress difference between the alloy and overlayer structures are responsible for the stabilization of domain patterns9,10.
The behaviour of the patterns at fixed coverage and varying temperature is also qualitatively consistent with theory3. For both droplets and stripes, the feature size decreases with increasing temperature. The most likely reason for this is a reduction in the domain-boundary free energy with increasing temperature. For droplets and inverted droplets, reducing the temperature also allows improvement in the long-range order. Figure 1h is a LEEM image showing the degree of long-range order that can be obtained in the inverted-droplet phase.
If self-assembled structures are to be useful as nanostructure templates, two-dimensional, self-assembled patterns need to be stable at room temperature and resistant to air exposure. This is the case for our Pb/Cu system. Features that correspond to lead-overlayer droplets can be clearly seen in atomic-force-microscopy images taken after cooling to room temperature and 2 hours exposure to air (Fig. 1i).
References
McConnell, H. M. Annu. Rev. Phys. Chem. 42, 171–195 (1991).
Seul, M. & Andelman, D. Science 267, 476–482 (1995).
Hurley, M. M. & Singer, S. J. Phys. Rev. B 46, 5783–5786 (1992).
Sagui, C. & Desai, R. C. Phys. Rev. E 49, 2225–2244 (1994).
Ng, K.-O & Vanderbilt, D. Phys. Rev. B 52, 2177–2183 (1995).
Henrion, J. & Rhead, G. E. Surf. Sci. 29, 20–36 (1972).
Meyer, G., Michailov, M. & Henzler, M. Surf. Sci. 202, 125–141 (1988).
Nagl, C., Haller, O., Platzgummer, E., Schmid, M. & Varga, P. Surf. Sci. 321, 237–248 (1994).
Marchenko, V. I. JETP Lett. 33, 381–383 (1981).
Alerhand, O. L., Vanderbilt, D., Meade, R. D. & Joannopoulos, J. D. Phys. Rev. Lett. 61, 1973–1976 (1988).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plass, R., Last, J., Bartelt, N. et al. Self-assembled domain patterns. Nature 412, 875 (2001). https://doi.org/10.1038/35091143
Issue Date:
DOI: https://doi.org/10.1038/35091143
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.