Abstract
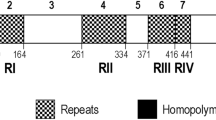
The malarial parasite Plasmodium vivax causes disease in humans, including chronic infections and recurrent relapses, but the course of infection is rarely fatal1,2, unlike that caused by Plasmodium falciparum. To investigate differences in pathogenicity between P. vivax and P. falciparum, we have compared the subtelomeric domains in the DNA of these parasites. In P. falciparum, subtelomeric domains are conserved and contain ordered arrays of members of multigene families, such as var3,4,5, rif6,7 and stevor8, encoding virulence determinants of cytoadhesion and antigenic variation. Here we identify, through the analysis of a continuous 155,711-base-pair sequence of a P. vivax chromosome end, a multigene family called vir, which is specific to P. vivax. The vir genes are present at about 600–1,000 copies per haploid genome and encode proteins that are immunovariant in natural infections, indicating that they may have a functional role in establishing chronic infection through antigenic variation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
David, P. H., del Portillo, H. A. & Mendis, K. N. Plasmodium vivax malaria: parasite biology defines potential targets for vaccine development. Biol. Cell 64, 251–260 (1988).
Galinski, M. R. & Barnwell, J. W. Plasmodium vivax: merozoites, invasion of reticulocytes and considerations for malaria vaccine development. Parasitol. Today 12, 20–29 (1996).
Baruch, D. I. et al. Cloning of the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell 82, 77–87 (1995).
Smith, J. D. et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82, 101–110 (1995).
Su, X. et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82, 89–100 (1995).
Kyes, S., Rowe, A. J., Kriek, N. & Newbold, C. I. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum. Proc. Natl Acad. Sci. USA 96, 9333–9338 (1999).
Fernandez, V., Hommel, M., Chen, Q., Hagblom, P. & Wahlgren, M. Small, clonally variant antigens expressed on the surface of Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J. Exp. Med. 190, 1393–1403 (1999).
Cheng, Q. et al. stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol. Biochem. Parasitol. 97, 161–176 (1998).
Golenda, C. F., Li, J. & Rosenberg, R. Continuous in vitro propagation of the malaria parasite Plasmodium vivax. Proc. Natl Acad. Sci. USA 94, 6786–6791 (1997).
Camargo, A. A., Fischer, K., Lanzer, M. & del Portillo, H. A. Construction and characterization of a Plasmodium vivax genomic library in yeast artificial chromosomes. Genomics 42, 467–473 (1997).
Ponzi, M., Pace, T., Dore, F. & Frontali, C. Identification of a telomeric DNA sequence in Plasmodium berghei. EMBO J. 4, 2991–2995 (1985).
Vernick, K. D. & McCutchan, T. F. Sequence and structure of a Plasmodium falciparum telomere. Mol. Biochem. Parasitol. 28, 85–94 (1988).
McCutchan, T. F., Dame, J. B., Miller, L. H. & Barnwell, J. Evolutionary relatedness of Plasmodium species as determined by the structure of DNA. Science 225, 808–811 (1984).
Gardner, M. J. et al. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum. Science 282, 1126–1132 (1998).
Bowman, S. et al. The complete nucleotide sequence of chromosome 3 of Plasmodium falciparum. Nature 400, 532–538 (1999).
The C. elegans sequencing consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science 282, 2012–2018 (1998).
Al-Khedery, B., Barnwell, J. W. & Galinski, M. R. Antigenic variation in malaria: a 3′ genomic alteration associated with the expression of a P. knowlesi variant antigen. Mol. Cell 3, 131–141 (1999).
Persson, B. Bioinformatics in protein analysis. EXS 88, 215–131 (2000).
Mendis, K. N., Ihalamulla, R. I. & David, P. H. Diversity of Plasmodium vivax-induced antigens on the surface of infected human erythrocytes. Am. J. Trop. Med. Hyg. 38, 42–46 (1988).
Freitas-Junior, L. H. et al. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature 407, 1018–1022 (2000).
Vaudin, M. et al. The construction and analysis of M13 libraries prepared from YAC DNA. Nucleic Acids Res. 23, 670–674 (1995).
Kyes, S., Pinches, R. & Newbold, C. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol Biochem. Parasitol. 105, 311–315 (2000).
Levitus, G. et al. Characterization of naturally acquired human IgG responses against the N-terminal region of the merozoite surface protein 1 of Plasmodium vivax. Am. J. Trop. Med. Hyg. 51, 68–76 (1994).
Hall, R. et al. Major surface antigen gene of a human malaria parasite cloned and expressed in bacteria. Nature 311, 379–382 (1984).
Oliveira, C. I. et al. Antigenic properties of the Merozoite Surface Protein 1 gene of Plasmodium vivax. Vaccine 17, 2959–2968 (1999).
Voller, A. & O'Neill, P. O. Immunofluorescence method suitable for large scale application to malaria. Bull. World Health Organ. 45, 524–529 (1971).
Acknowledgements
We thank all the patients who participated in this study; J. d'Arc Neves for collecting blood samples; A. Craig for helping in the initial liasion with the Sanger Centre; M. Quail and the subcloning group; members of team 23 and other members of the Pathogen Unit at the Sanger Centre for their help in sequence generation and analysis; N. Hall for the creation of the web pages; Y. Cully for the graphics; and H. Bujard for encouragement throughout this work. This work was supported by the the Deutsche Forschungsgemeinschaft (to M.L.) and the European Commission (to M.L. and H.A.P).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
del Portillo, H., Fernandez-Becerra, C., Bowman, S. et al. A superfamily of variant genes encoded in the subtelomeric region of Plasmodium vivax. Nature 410, 839–842 (2001). https://doi.org/10.1038/35071118
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35071118
This article is cited by
-
Humoral and cellular immune response to Plasmodium vivax VIR recombinant and synthetic antigens in individuals naturally exposed to P. vivax in the Republic of Korea
Malaria Journal (2021)
-
Genetic polymorphism of the extracellular region in surface associated interspersed 1.1 gene of Plasmodium falciparum field isolates from Thailand
Malaria Journal (2021)
-
Analysis of pir gene expression across the Plasmodium life cycle
Malaria Journal (2021)
-
A suitable RNA preparation methodology for whole transcriptome shotgun sequencing harvested from Plasmodium vivax-infected patients
Scientific Reports (2021)
-
Plasma-derived extracellular vesicles from Plasmodium vivax patients signal spleen fibroblasts via NF-kB facilitating parasite cytoadherence
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.