Abstract
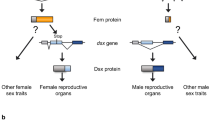
Sexually dimorphic abdominal pigmentation and segment morphology evolved recently in the melanogaster species group of the fruitfly Drosophila . Here we show that these traits are controlled by the bric-a-brac (bab) gene, which integrates regulatory inputs from the homeotic and sex-determination pathways. bab expression is modulated segment- and sex-specifically in sexually dimorphic species, but is uniform in sexually monomorphic species. We suggest that bab has an ancestral homeotic function, and that regulatory changes at the bab locus played a key role in the evolution of sexual dimorphism. Pigmentation patterns specified by bab affect mating preferences, suggesting that sexual selection has contributed to the evolution of bab regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Warren, R., Nagy, L., Selegue, J., Gates, J. & Carroll, S. Evolution of homeotic gene regulation and function in flies and butterflies. Nature 372, 458– 461 (1994).
Burke, A. C., Nelson, C. E., Morgan, B. A. & Tabin, C. Hox genes and the evolution of vertebrate axial morphology. Development 121, 333–346 (1995).
Averof, M. & Akam, M. Hox genes and the diversification of insect-crustacean body plans. Nature 376, 420–423 (1995).
Brakefield, P. M. et al. Development, plasticity and evolution of butterfly eyespot patterns. Nature 384, 236– 242 (1996).
Averof, M. & Patel, N. H. Crustacean appendage evolution associated with changes in Hox gene expression. Nature 388, 682–686 (1997).
Stern, D. L. A role of Ultrabithorax in morphological differences between Drosophila species. Nature 396, 463– 466 (1998).
Sucena, E. & Stern, D. L. Divergence of larval morphology between Drosophila sechellia and its sibling species caused by cis-regulatory evolution of ovo/shaven-baby. Proc. Natl Acad. Sci. USA 97, 4530–4534 ( 2000).
True, J. R., Stam, L. F., Zeng, Z. -B. & Laurie, C. C. Quantitative genetic analysis of divergence in male secondary sexual traits between Drosophila simulans and Drosophila mauritiana. Evolution 51, 816–832 ( 1997).
Gurganus, M. C., Nuzhdin, S. V., Leips, J. W. & MacKay, T. F. High-resolution mapping of quantitative trait loci for sternopleural bristle number in Drosophila melanogaster. Genetics 152, 1535–1604 (1999).
Lyman, R. F., Lai, C. & MacKay, T. F. Linkage disequilibrium mapping of molecular polymorphisms at the scabrous locus associated with naturally occurring variation in bristle number in Drosophila melanogaster. Genet. Res. 74, 303–311 ( 1999).
Anderson, M. Sexual Selection (Princeton Univ. Press, Princeton, 1994).
Eberhard, W. G. Copulatory courtship and cryptic female choice in insects. Biol. Rev. 66, 1–31 ( 1991).
Kopp, A. & Duncan, I. Control of cell fate and polarity in the adult abdominal segments of Drosophila by optomotor-blind . Development 124, 3703– 3714 (1997).
Sanchez-Herrero, E., Vernos, I., Marco, R. & Morata, G. Genetic organization of the Drosophila bithorax complex. Nature 313, 108–113 (1985).
Duncan, I. M. The bithorax complex. Annu. Rev. Genet. 21, 285–319 (1987).
Celniker, S. E., Sharma, S., Keelan, D. J. & Lewis, E. B. The molecular genetics of the bithorax complex of Drosophila: cis-regulation in the Abdominal-B domain. EMBO J. 9, 4277–4286 (1990).
Crosby, M. A., Lundquist, E. A., Tautvydas, R. M. & Johnson, J. The 3′ regulatory region of the Abdominal-B gene: genetic analysis supports a model of reiterated and interchangeable regulatory elements. Genetics 134, 809–824 ( 1993).
Hopmann, R., Duncan, D. & Duncan, I. Transvection in the iab-5,6,7 region of the bithorax complex of Drosophila: homology independent interactions in trans . Genetics 139, 815– 833 (1994).
Baker, B. S. & Ridge, K. A. Sex and the single cell. I. On the action of major loci affecting cell determination in Drosophila melanogaster . Genetics 94, 383– 423 (1980).
Cline, T. W. & Meyer, B. J. Vive al difference: males vs. females in flies vs. worms. Annu. Rev. Genet. 30, 637–702 (1996).
McKeown, M. Sex differentiation: the role of alternative splicing. Curr. Opin. Genet. Dev. 2, 299–303 ( 1992).
Burtis, K. C. The regulation of sex determination and sexually dimorphic differentiation in Drosophila. Curr. Opin. Cell Biol. 5, 1006–1014 (1993).
Jursnich, V. A. & Burtis, K. C. A positive role in differentiation for the male doublesex protein of Drosophila. Dev. Biol. 155, 235–249 (1993).
Waterbury, J. A., Jackson, L. L. & Schedl, P. Analysis of the doublesex female protein in Drosophila melanogaster: Role in sexual differentiation and behavior and dependence on intersex. Genetics 152, 1653– 1667 (1999).
Robertson, A., Briscoe, D. A. & Louw, J. H. Variation in abdomen pigmentation in Drosophila melanogaster females. Genetica 47, 73 –76 (1977).
Godt, D., Couderc, J. -L., Cramton, S. E. & Laski, F. Pattern formation in the limbs of Drosophila: bric a brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development 119, 799–812 (1993).
Pointud, J. C. Fly base personal communications report 〈http://flybase.bio.indiana.edu/.bin/fbpcq.html?FBrf0104845〉 (1998).
Nagoshi, R. N. & Baker, B. S. Regulation of sex-specific RNA splicing at the Drosophila doublesex gene: cis -acting mutations in exon sequences alter sex-specific RNA splicing patterns. Genes Dev. 4, 89–97 (1990).
Burtis, K. C., Coschigano, K. T., Baker, B. S. & Wensink, P. C. The Doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J. 10, 2577–2582 (1991).
Coshigano, K. T. & Wensink, P. C. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 7, 42– 54 (1993).
Bock, I. R. Taxonomy of the Drosophila bipectinata species complex. Univ. Texas Publ. 7103, 273–280 (1971).
Ohnishi, S. & Watanabe, T. K. Genetic analysis of color dimorphism in the Drosophila montium subgroup. Jpn J. Genet. 60, 355–358 (1985).
Fisher, R. A. The Genetical Theory Of Natural Selection (Dover, New York, 1958).
Carson, H. L. in Ecological Genetics: The Interface (ed. Brussard, P. F.) 93– 107 (Springer, New York, 1978).
Lande, R. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721– 3725 (1981).
Kirkpatrick, M. Sexual selection and the evolution of female choice. Evolution 36, 1–12 (1982 ).
Carson, H. L. in Evolutionary Genetics (eds Singh, R. S. & Krimbas, C. B.) 495–512 (Cambridge Univ. Press, Cambridge, 1999).
Holland, B. & Rice, W. R. Chase-away sexual selection: antagonistic seduction versus resistance. Evolution 52, 1–7 (1998).
Cordts, R. & Partridge, L. Courtship reduces longevity of male Drosophila melanogaster. Anim. Behav. 52, 269–278 (1996).
Kopp, A. & Duncan, I. Technical tips for analyzing gene expression in the pupal abdomen. Dros. Info. Serv. (in the press).
Bender, W. & Hudson, A. P element homing to the Drosophila bithorax complex. Development 127, 3981–3992 (2000).
Kellerman, K. A. Mutations affecting the stability of the fushi tarazu protein of Drosophila. Genes Dev. 4, 1936– 1950 (1990).
Bock, I. R. & Wheeler, M. R. The Drosophila melanogaster species group. Univ. Texax Publ. 7213, 1–102 (1972).
Bock, I. R. Current status of the Drosophila melanogaster species-group (Diptera). Syst. Entomol. 5, 341– 356 (1980).
Pelandakis, M. & Solignac, M. Molecular phylogeny of Drosophila based on ribosomal RNA sequences. J. Mol. Evol. 37, 525–543 ( 1993).
Toda, M. J. Drosophilidae (Diptera) in Myanmar (Burma). VII. The Drosophila melanogaster species-group, excepting the D. montium species-subgroup. Orient. Insects 25, 69–94 (1991).
Russo, C. A. M., Takezaki, N. & Nei, M. Molecular phylogeny and divergence times of drosophilid species. Mol. Biol. Evol. 12, 391– 404 (1995).
Acknowledgements
We thank F. Laski for bab stocks and antibodies; D. Godt for UAS- bab lines; W. Bender, S. Celniker and E. Sanchez-Herrero for the Abd-B enhancer trap and antibodies; J. David, Y. Fuyama, M. Kimura, J. Roote and the Bowling Green stock centre for various Drosophila species; L. Olds for the artwork; B. Holland, S. Nuzhdin, M. Servedio and J. True and T. Wittkopp for discussions. A.K. is a Howard Hughes Medical Institute fellow of the Life Sciences Research Foundation; I.D. is supported by an NIH grant; S.B.C. is an investigator at the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Kopp, A., Duncan, I. & Carroll, S. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 408, 553–559 (2000). https://doi.org/10.1038/35046017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35046017
This article is cited by
-
The function and evolution of a genetic switch controlling sexually dimorphic eye differentiation in honeybees
Nature Communications (2023)
-
Thermal plasticity of wing size and wing spot size in Drosophila guttifera
Development Genes and Evolution (2023)
-
Candidate genes associated with color morphs of female-limited polymorphisms of the damselfly Ischnura senegalensis
Heredity (2019)
-
The mimetic wing pattern of Papilio polytes butterflies is regulated by a doublesex-orchestrated gene network
Communications Biology (2019)
-
Asymmetric interactions between doublesex and tissue- and sex-specific target genes mediate sexual dimorphism in beetles
Nature Communications (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.