Abstract
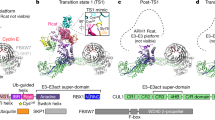
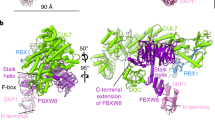
F-box proteins are members of a large family that regulates the cell cycle, the immune response, signalling cascades and developmental programmes by targeting proteins, such as cyclins, cyclin-dependent kinase inhibitors, IκBα and β-catenin, for ubiquitination (reviewed in refs 1,2,3). F-box proteins are the substrate-recognition components of SCF (Skp1–Cullin–F-box protein) ubiquitin-protein ligases4,5. They bind the SCF constant catalytic core by means of the F-box motif interacting with Skp1, and they bind substrates through their variable protein–protein interaction domains6. The large number of F-box proteins is thought to allow ubiquitination of numerous, diverse substrates6. Most organisms have several Skp1 family members, but the function of these Skp1 homologues and the rules of recognition between different F-box and Skp1 proteins remain unknown. Here we describe the crystal structure of the human F-box protein Skp2 bound to Skp1. Skp1 recruits the F-box protein through a bipartite interface involving both the F-box and the substrate-recognition domain. The structure raises the possibility that different Skp1 family members evolved to function with different subsets of F-box proteins, and suggests that the F-box protein may not only recruit substrate, but may also position it optimally for the ubiquitination reaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Patton, E. E., Willems, A. R. & Tyers, M. Combinatorial control in ubiquitin-dependent proteolysis: don’t Skp the F-box hypothesis. Trends Genet. 14, 236–243 (1998).
Koepp, D. M., Harper, J. W. & Elledge, S. J. How the cyclin became a cyclin: regulated proteolysis in the cell cycle. Cell 97, 431– 434 (1999).
Deshaies, R. J. SCF and Cullin/Ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15, 435–467 ( 1999).
Skowyra, D., Craig, K. L., Tyers, M., Elledge, S. J. & Harper, J. W. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91, 209–219 (1997).
Feldman, R. M., Correll, C. C., Kaplan, K. B. & Deshaies, R. J. A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91, 221–230 (1997).
Bai, C. et al. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86 , 263–274 (1996).
Zhang, H., Kobayashi, R., Galaktionov, K. & Beach, D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell 82, 915–925 (1995).
Sutterlüty, H. et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nature Cell Biol. 1, 207 –214 (1999).
Carrano, A. C., Eytan, E., Hershko, A. & Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biol. 1, 193–199 ( 1999).
Tsvetkov, L. M., Yeh, K. H., Lee, S. J., Sun, H. & Zhang, H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 9 , 661–664 (1999).
Nakayama, K. et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 19, 2069–2081 ( 2000).
Aravind, L. & Koonin, E. V. Fold prediction and evolutionary analysis of the POZ domain: structural and evolutionary relationship with the potassium channel tetramerization domain. J. Mol. Biol. 285, 1353–1361 (1999).
Kobe, B. & Deisenhofer, J. Crystal structure of porcine ribonuclease inhibitor, a protein with leucine-rich repeats. Nature 366, 751–756 ( 1993).
Li, F. N. & Johnston, M. Grr1 of Saccharomyces cerevisiae is connected to the ubiquitin proteolysis machinery through Skp1: coupling glucose sensing to gene expression and the cell cycle. EMBO J. 16, 5629–5638 ( 1997).
Zhou, P. & Howley, P. M. Ubiquitination and degradation of the substrate recognition subunits of SCF ubiquitin-protein ligases. Mol. Cell 2, 571–580 ( 1998).
Connelly, C. & Hieter, P. Budding yeast SKP1 encodes an evolutionarily conserved kinetochore protein required for cell cycle progression. Cell 86, 275–285 ( 1996).
Russell, I. D., Grancell, A. S. & Sorger, P. K. The unstable F-box protein p58-Ctf13 forms the structural core of the CBF3 kinetochore complex. J. Cell Biol. 145, 933–950 (1999).
Lisztwan, J. et al. Association of human CUL-1 and ubiquitin-conjugating enzyme CDC34 with the F-box protein p45(SKP2): evidence for evolutionary conservation in the subunit composition of the CDC34-SCF pathway. EMBO J. 17, 368–383 (1998).
Costanzo, M. C. et al. The yeast proteome database (YPD) and Caenorhabditis elegans proteome database (WormPD): comprehensive resources for the organization and comparison of model organism protein information. Nucleic Acids Res. 28, 73–76 ( 2000).
Rubin, G. M. et al. Comparative genomics of the eukaryotes. Science 287, 2204–2215 ( 2000).
Lonergan, K. M. et al. Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol. Cell. Biol. 18, 732– 41 (1998).
Kamura, T. et al. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science 284, 657– 661 (1999).
Stebbins, C. E., Kaelin, W. G. Jr & Pavletich, N. P. Structure of the VHL-ElonginC-ElonginB complex: implications for VHL tumor suppressor function. Science 284, 455–461 ( 1999).
Shirane, M. et al. Down-regulation of p27(Kip1) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J. Biol. Chem. 274, 13886–13893 (1999).
Spencer, E., Jiang, J. & Chen, Z. J. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 13, 284–294 (1999).
Kobe, B. & Deisenhofer, J. A structural basis of the interactions between leucine-rich repeats and protein ligands. Nature 374, 183–186 (1995).
Price, S. R., Evans, P. R. & Nagai, K. Crystal structure of the spliceosomal U2B′′–U2A′ protein complex bound to a fragment of U2 small nuclear RNA. Nature 394, 645–650 ( 1998).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 176, 307–326 ( 1997).
Collaborative Computational Project B. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 ( 1994).
Brünger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D 54, 905–921 (1998).
Acknowledgements
We thank H. Erdument-Bromage for N-terminal sequence and mass spectroscopic analysis; P. Sorger for communication of results before publication; A. Ansari, P. Murray and members of the Pavletich lab for discussions; C. Murray for administrative assistance; and the staff of the National Synchotron Light Source X9B beamline and of the Cornell High Energy Synchotron Source MacChess for help with data collection. B.A.S. was supported by a Foundation for Advanced Cancer Studies fellowship from the Life Sciences Research Foundation. This work was supported by the NIH, the Howard Hughes Medical Institute, the Dewitt Wallace Foundation, the Samuel and May Rudin Foundation, the Human Frontiers Science Program and the Welch Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Howard Hughes Medical Institute, Baylor College of Medicine, Houston, 77030, Texas, USA
Supplementary information
Supplementary Information
Supplementary Information (PDF 33 kb)
Rights and permissions
About this article
Cite this article
Schulman, B., Carrano, A., Jeffrey, P. et al. Insights into SCF ubiquitin ligases from the structure of the Skp1–Skp2 complex. Nature 408, 381–386 (2000). https://doi.org/10.1038/35042620
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35042620
This article is cited by
-
Identification, Genomic Organization, and Comprehensive Expression Analysis Reveals the Implication of Cicer arietinum SKP1-like Genes in Abiotic Stress
Journal of Plant Growth Regulation (2023)
-
Inhibitors Targeting the F-BOX Proteins
Cell Biochemistry and Biophysics (2023)
-
The roles of a novel CDKB/KRP/FB3 cell cycle core complex in rice gametes and initiation of embryogenesis
Plant Reproduction (2023)
-
Ubiquitination of NF-κB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and paclitaxel resistance
Cell Death & Differentiation (2022)
-
NUMB facilitates autophagy initiation through targeting SCFβ-TrCP2 complex
Cell Death & Differentiation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.