Abstract
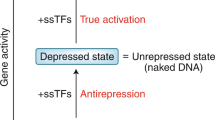
High levels of gene transcription by RNA polymerase II depend on high rates of transcription initiation and reinitiation. Initiation requires recruitment of the complete transcription machinery to a promoter, a process facilitated by activators and chromatin remodelling factors. Reinitiation probably occurs through a different pathway1. After initiation, a subset of the transcription machinery remains at the promoter, forming a platform for assembly of a second transcription complex2,3,4. Here we describe the isolation of a reinitiation intermediate that includes transcription factors TFIID, TFIIA, TFIIH, TFIIE and Mediator. This intermediate can act as a scaffold for formation of a functional reinitiation complex. Formation of this scaffold is dependent on ATP and TFIIH. The scaffold is stabilized in the presence of the activator Gal4–VP16, but not Gal4–AH, suggesting a new role for some activators and Mediator in promoting high levels of transcription.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hahn, S. Activation and the role of reinitiation in the control of transcription by RNA polymerase II. Cold Spring Harb. Symp. Quant. Biol. 63, 181–188 (1998).
Roberts, S. G., Choy, B., Walker, S. S., Lin, Y. S. & Green, M. R. A role for activator-mediated TFIIB recruitment in diverse aspects of transcriptional regulation. Curr. Biol. 5, 508–516 (1995).
Zawel, L., Kuman, K. P. & Reinberg, D. Recycling of the general transcription factors during RNA polymerase II transcription. Genes Dev. 9, 1479–1490 (1995).
Sandaltzopoulos, R. & Becker, P. B. Heat shock factor increases the reinitiation rate from potentiated chromatin templates. Mol. Cell. Biol. 18, 361–367 (1998).
Ranish, J. A., Yudkovsky, N. & Hahn, S. Intermediates in formation and activity of the RNA polymerase II preinitiation complex: holoenzyme recruitment and a postrecruitment role for the TATA box and TFIIB. Genes Dev. 13, 49–63 (1999).
Kim, Y. J., Bjorklund, S., Li, Y., Sayre, M. H. & Kornberg, R. D. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA Polymerase II. Cell 77, 599–608 (1994).
Koleske, A. J. & Young, R. A. An RNA polymerase II holoenzyme responsive to activators. Nature 368, 466 –469 (1994).
Jiang, Y. & Gralla, J. D. Uncoupling of initiation and reinitiation rates during HeLa RNA polymerase II transcription in vitro. Mol. Cell. Biol. 13, 4572–4577 (1993).
Svejstrup, J. Q. et al. Evidence for a mediator cycle at the initiation of transcription. Proc. Natl Acad. Sci. USA 94, 6075– 6078 (1997).
Kraus, W. L. & Kadonaga, J. T. p300 and estrogen receptor cooperatively activate transcription via differential enhancement of initiation and reinitiation. Genes Dev. 12, 331–342 (1998).
Sheridan, P. L., Mayall, T. P., Verdin, E. & Jones, K. A. Histone acetyltransferases regulate HIV-1 enhancer activity in vitro. Genes Dev. 11, 3327–3340 (1997).
Ho, S. N., Biggar, S. R., Spencer, D. M., Schreiber, S. L. & Crabtree, G. R. Dimeric ligands define a role for transcriptional activation domains in reinitiation. Nature 382, 822–826 ( 1996).
Stringer, K. F., Ingles, C. J. & Greenblatt, J. Direct and selective binding of an acidic transcriptional activation domain to the TATA-box factor TFIID. Nature 345, 783–786 (1990).
Ozer, J. et al. Molecular cloning of the small (γ) subunit of human TFIIA reveals functions critical for activated transcription. Genes Dev. 8, 2324–2335 ( 1994).
Koh, S. S., Ansari, A. Z., Ptashne, M. & Young, R. A. An activator target in the RNA polymerase II holoenzyme. Mol. Cell 1, 895–904 ( 1998).
Lee, Y. C., Park, J. M., Min, S., Han, S. J. & Kim, Y. J. An activator binding module of yeast RNA polymerase II holoenzyme. Mol. Cell. Biol. 19, 2967– 2976 (1999).
Yean, D. & Gralla, J. Transcription reinitiation rate: a special role for the TATA box. Mol. Cell. Biol. 17 , 3809–3816 (1997).
Klein, C. & Struhl, K. Increased recruitment of TATA-binding protein to the promoter by transcriptional activation domains in vivo. Science 266, 280–282 ( 1994).
Xiao, H., Friesen, J. D. & Lis, J. T. Recruiting TATA-binding protein to a promoter: transcription activation without an upstream activator. Mol. Cell. Biol. 15, 5757–5761 (1995).
Farrell, S., Simkovich, N., Wu, Y., Barberis, A. & Ptashne, M. Gene activation by recruitment of the RNA polymerase II holoenzyme. Genes Dev. 10, 2359– 2367 (1996).
Keaveney, M. & Struhl, K. Activator-mediated recruitment of the RNA polymerase II machinery is the predominant mechanism for transcriptional activation in yeast. Mol. Cell 1, 917– 924 (1998).
Hawley, D. K. & Roeder, R. G. Functional steps in transcription initiation and reinitiation from the major late promoter in a HeLa nuclear extract. J. Biol. Chem. 262, 3452– 3461 (1987).
Holstege, F. C. et al. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95, 717–728 (1998).
Guzder, S. N. et al. DNA repair gene RAD3 of S. cerevisiae is essential for transcription by RNA polymerase II. Nature 367, 91– 94 (1994).
Cismowski, M. J., Laff, G. M., Solomon, M. J. & Reed, S. I. KIN28 encodes a C-terminal domain kinase that controls mRNA transcription in S. cerevisiae but lacks cyclin-dependent kinase-activating kinase (CAK) activity. Mol. Cell. Biol. 15, 2983– 2992 (1995).
Kang, J. J., Auble, D. T., Ranish, J. A. & Hahn, S. Analysis of the yeast transcription factor TFIIA: distinct functional regions and a polymerase II-specific role in basal and activated transcription. Mol Cell Biol 15, 1234-1243 ( 1995).
Ranish, J. A. & Hahn, S. The yeast general transcription factor TFIIA is composed of two polypeptide subunits. J. Biol. Chem. 266, 19320–19327 (1991).
Acknowledgements
We thank members of the Hahn and Reeder laboratories for helpful discussions, and A. Krumm, S. Parkhurst and R. Reeder for comments on the manuscript. We also thank L. Prakash for providing the Rad3ts strain, M. Solomon for providing the Kin28ts strain, D. Reinberg for TFIIE antibodies and H. Sakurai for providing the Tfa1ts strain and antibodies to Gal11. This work was supported by grants from the NIH to S.H. and an NIH training grant to N.Y. S.H. is an associate investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Yudkovsky, N., Ranish, J. & Hahn, S. A transcription reinitiation intermediate that is stabilized by activator . Nature 408, 225–229 (2000). https://doi.org/10.1038/35041603
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35041603
This article is cited by
-
The Mediator kinase module enhances polymerase activity to regulate transcriptional memory after heat stress in Arabidopsis
The EMBO Journal (2024)
-
RNA polymerase II associates with active genes during DNA replication
Nature (2023)
-
The Mediator complex as a master regulator of transcription by RNA polymerase II
Nature Reviews Molecular Cell Biology (2022)
-
Structural insights into nuclear transcription by eukaryotic DNA-dependent RNA polymerases
Nature Reviews Molecular Cell Biology (2022)
-
Beyond the canonical role of TFIIB in eukaryotic transcription
Current Genetics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.