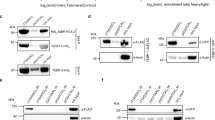
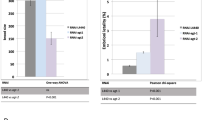
Abstract
The germ line is an immortal cell lineage that is passed indefinitely from one generation to the next. To identify the genes that are required for germline immortality, we isolated Caenorhabditis elegans mutants with mortal germ lines—worms that can reproduce for several healthy generations but eventually become sterile. One of these mortal germline (mrt ) mutants, mrt-2, exhibits progressive telomere shortening and accumulates end-to-end chromosome fusions in later generations, indicating that the MRT-2 protein is required for telomere replication. In addition, the germ line of mrt-2 is hypersensitive to X-rays and to transposon activity. Therefore, mrt-2 has defects in responding both to damaged DNA and to normal double-strand breaks present at telomeres. mrt-2 encodes a homologue of a checkpoint gene that is required to sense DNA damage in yeast. These results indicate that telomeres may be identified as a type of DNA damage and then repaired by the telomere-replication enzyme telomerase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wylie, C. Germ cells. Cell 96, 165–174 (1999).
Strome, S. & Wood, W. B. Immunofluorescence visualization of germ-line-specific cytoplasmic granules in embryos, larvae, and adults of Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 79, 1558–1562 (1982).
Hodgkin, J. & Doniach, T. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics 146, 149–164 (1997).
Blasco, M. A. et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell 91, 25– 34 (1997).
Nakamura, T. M., Cooper, J. P. & Cech, T. R. Two modes of survival of fission yeast without telomerase. Science 282, 493–496 (1998).
Naito, T., Matsuura, A. & Ishikawa, F. Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nature Genet. 20, 203–206 (1998).
Greider, C. W. & Blackburn, E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51, 887– 898 (1987).
Nugent, C. I. & Lundblad, V. The telomerase reverse transcriptase: components and regulation. Genes Dev. 12, 1073–1085 (1998).
Lundblad, V. & Szostak, J. W. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell 57, 633–643 (1989).
Lee, H. W. et al. Essential role of mouse telomerase in highly proliferative organs. Nature 392, 569–574 (1998).
Palladino, F. SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell 75, 543– 555 (1993).
Porter, S. E., Greenwell, P. W., Ritchie, K. B. & Petes, T. D. The DNA-binding protein Hdf1p (a putative Ku homologue) is required for maintaining normal telomere length in Saccharomyces cerevisiae. Nucleic Acids Res. 24, 582–585 ( 1996).
Boulton, S. J. & Jackson, S. P. Identification of a Saccharomyces cerevisiae Ku80 homologue: roles in DNA double strand break rejoining and in telomeric maintenance. Nucleic Acids Res. 24 , 4639–4648 (1996).
Boulton, S. J. & Jackson, S. P. Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 17, 1819 –1828 (1998).
Nugent, C. I. et al. Telomere maintenance is dependent on activities required for end repair of double-strand breaks. Curr. Biol. 8, 657–660 (1998).
Vaziri, H. et al. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 16, 6018–6033 (1997).
Sprung, C. N., Bryan, T. M., Reddel, R. R. & Murnane, J. P. Normal telomere maintenance in immortal ataxia telangiectasia cell lines. Mutat. Res. 379, 177–184 (1997).
Greenwell, P. W. et al. TEL1, a gene involved in controlling telomere length in S. cerevisiae, is homologous to the human ataxia telangiectasia gene. Cell 82, 823–829 (1995).
Dahlen, M., Olsson, T., Kanter-Smoler, G., Ramne, A. & Sunnerhagen, P. Regulation of telomere length by checkpoint genes in Schizosaccharomyces pombe. Mol. Biol. Cell. 9, 611–621 ( 1998).
Ritchie, K. B., Mallory, J. C. & Petes, T. D. Interactions of TLC1 (which encodes the RNA subunit of telomerase), TEL1, and MEC1 in regulating telomere length in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 19, 6065–6075 ( 1999).
Anderson, P. Mutagenesis. Methods Cell Biol. 48, 31– 58 (1995).
Hodgkin, J. A., Horvitz, H. R. & Brenner, S. Nondisjunction mutants of the nematode C. elegans . Genetics 91, 67–94 (1979).
Plasterk, R. H. A. & van Leunen, H. G. A. M. Transposons. in C. elegans II (eds Riddle, D. L., Blumenthal, T., Meyer, B. J. & Priess, J. R.) 97–116 (Cold Spring Harbor Laboratory Press, Plainview, 1997).
Herman, R. K., Kari, C. K. & Hartman, P. S. Dominant X-chromosome nondisjunction mutants of Caenorhabditis elegans. Genetics 102, 379–400 (1982).
McClintock, B. The stability of broken ends of chromosomes of Zea mays. Genetics 26, 234–282 ( 1941).
Albertson, D. G. & Thomson, J. N. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans . Chromosome Res. 1, 15– 26 (1993).
Wicky, C. et al. Telomeric repeats (TTAGGC)n are sufficient for chromosome capping function in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 93, 8983–8988 ( 1996).
van Steensel, B., Smogorzewska, A. & de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 92, 401–413 (1998).
d'Adda di Fagagna, F. et al. Functions of poly(ADP-ribose) polymerase in controlling telomere length and chromosomal stability. Nature Genet. 23, 76–80 (1999).
Lundblad, V. & Blackburn, E. H. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell 73, 347–360 (1993).
Dean, F. B., Lian, L. & O'Donnell, M. cDNA cloning and gene mapping of human homologs for Schizosaccharomyces pombe rad17, rad1, and hus1 and cloning of homologs from mouse, Caenorhabditis elegans, and Drosophila melanogaster . Genomics 54, 424– 436 (1998).
Bluyssen, H. A. et al. A human and mouse homolog of the Schizosaccharomyces pombe rad1+ cell cycle checkpoint control gene. Genomics 54, 331–337 (1998).
al-Khodairy, F. & Carr, A. M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 11, 1343–1350 ( 1992).
Marathi, U. K. et al. RAD1, a human structural homolog of the Schizosaccharomyces pombe RAD1 cell cycle checkpoint gene. Genomics 54, 344–347 (1998).
Freire, R. et al. Human and mouse homologs of Schizosaccharomyces pombe rad1 (+) and Saccharomyces cerevisiae RAD17: linkage to checkpoint control and mammalian meiosis. Genes Dev. 12, 2560 –2573 (1998).
Weinert, T. A. & Hartwell, L. H. Cell cycle arrest of cdc mutants and specificity of the RAD9 checkpoint. Genetics 134 , 63–80 (1993).
Enoch, T., Carr, A. M. & Nurse, P. Fission yeast genes involved in coupling mitosis to completion of DNA replication. Genes Dev. 6, 2035– 2046 (1992).
Morrow, D. M., Tagle, D. A., Shiloh, Y., Collins, F. S. & Hieter, P. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell 82, 831–840 (1995).
Matsuura, A., Naito, T. & Ishikawa, F. Genetic control of telomere integrity in Schizosaccharomyces pombe, rad3(+) and tel1(+) are parts of two regulatory networks independent of the downstream protein kinases chk1(+) and cds1(+). Genetics 152, 1501–1512 (1999).
Sandell, L. L. & Zakian, V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell 75, 729–739 (1993).
Henderson, E. in Telomeres (eds Blackburn, E. H. & Greider, C. W.) 35– 68 (Cold Spring Harbor Laboratory Press, Plainview, 1995).
Griffith, J. D. et al. Mammalian telomeres end in a large duplex loop. Cell 97, 503–514 ( 1999).
Lydall, D. & Weinert, T. Yeast checkpoint genes in DNA damage processing: implications for repair and arrest. Science 270, 1488–1491 (1995).
Wellinger, R. J., Wolf, A. J. & Zakian, V. A. Saccharomyces telomeres acquire single-strand TG1-3 tails late in S phase. Cell 72, 51 –60 (1993).
Nugent, C. I., Hughes, T. R., Lue, N. F. & Lundblad, V. Cdc13p: a single-strand telomeric DNA-binding protein with a dual role in yeast telomere maintenance. Science 274, 249–252 (1996).
Mills, K. D., Sinclair, D. A. & Guarente, L. MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell 97, 609–620 (1999).
Martin, S. G., Laroche, T., Suka, N., Grunstein, M. & Gasser, S. M. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97, 621–633 (1999).
Fang, G. & Cech, T. R. Telomerase RNA localized in the replication band and spherical subnuclear organelles in hypotrichous ciliates. J. Cell Biol. 130, 243–253 (1995).
Chin, L. et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell 97, 527–538 ( 1999).
Sulston, J. & Hodgkin, J. in The Nematode Caenorhabditis elegans (ed. Wood, W. B.) 587–606 (Cold Spring Harbor Laboratory Press, Plainview, 1988).
Acknowledgements
We thank R. Hill, R. H. A. Plasterk and P. Kuwabara for advice and discussions; K. Van Auken for nob-1 (ct230); F. Müller and A. Coulson for plasmids; and A. Gartner, K. J. Patel, R. Harris, M. O'Reilly, D. Rhodes, V. P. C. C. Yu, A. Woollard, N. Hopper, M. Bickle, S. Sokol, and C. Milne for discussions and comments on this manuscript. Some strains were from the C. elegans Genetics Center (St. Paul, Minnesota), which is supported by the National Center for Research Resources. This work was supported by the Medical Research Council UK and the Howard Hughes Medical Institute. S.A. is a recipient of a Burroughs Wellcome Fund Hitchings-Elion Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, S., Hodgkin, J. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature 403, 159–164 (2000). https://doi.org/10.1038/35003120
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35003120
This article is cited by
-
Telomere DNA length regulation is influenced by seasonal temperature differences in short-lived but not in long-lived reef-building corals
Nature Communications (2023)
-
Nucleophagy delays aging and preserves germline immortality
Nature Aging (2022)
-
Somatic PMK-1/p38 signaling links environmental stress to germ cell apoptosis and heritable euploidy
Nature Communications (2022)
-
The arithmetic mean of what? A Cautionary Tale about the Use of the Geometric Mean as a Measure of Fitness
Biology & Philosophy (2022)
-
The double-stranded DNA-binding proteins TEBP-1 and TEBP-2 form a telomeric complex with POT-1
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.