Abstract
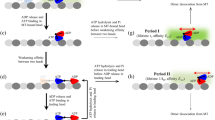
Kinesin is a two-headed, ATP-driven motor protein that moves processively along microtubules in discrete steps of 8 nm, probably by advancing each of its heads alternately in sequence1,2,3,4. Molecular details of how the chemical energy stored in ATP is coupled to mechanical displacement remain obscure. To shed light on this question, a force clamp was constructed, based on a feedback-driven optical trap capable of maintaining constant loads on single kinesin motors5. The instrument provides unprecedented resolution of molecular motion and permits mechanochemical studies under controlled external loads. Analysis of records of kinesin motion under variable ATP concentrations and loads revealed several new features. First, kinesin stepping appears to be tightly coupled to ATP hydrolysis over a wide range of forces, with a single hydrolysis per 8-nm mechanical advance. Second, the kinesin stall force depends on the ATP concentration. Third, increased loads reduce the maximum velocity as expected, but also raise the apparent Michaelis–Menten constant. The kinesin cycle therefore contains at least one load-dependent transition affecting the rate at which ATP molecules bind and subsequently commit to hydrolysis. It is likely that at least one other load-dependent rate exists, affecting turnover number. Together, these findings will necessitate revisions to our understanding of how kinesin motors function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Howard, J. Molecular motors: structural adaptations to cellular functions. Nature 389, 561–567 (1997).
Howard, J. The movement of kinesin along microtubules. Annu. Rev. Physiol. 58, 703–729 (1996).
Svoboda, K., Schmidt, C. F., Schnapp, B. J. & Block, S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727 (1993).
Howard, J., Hudspeth, A. J. & Vale, R. D. Movement of microtubules by single kinesin molecules. Nature 342, 154–158 (1989).
Visscher, K. & Block, S. M. Versatile optical traps with feedback control. Methods Enzymol. 298, 460–489 (1998).
Funatsu, T., Harada, Y., Tokunaga, M., Saito, K. & Yanagida, T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature 374, 555–559 (1995).
Ishijima, A. et al. Simultaneous observation of individual ATPase and mechanical events by a single myosin molecule during interaction with actin. Cell 92, 161–171 (1998).
Schnitzer, M. J. & Block, S. M. Kinesin hydrolyses one ATP per 8-nm step. Nature 388, 386–390 (1997).
Hua, W., Young, E. C., Fleming, M. L. & Gelles, J. Coupling of kinesin steps to ATP hydrolysis. Nature 388, 390–393 (1997).
Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. Cell 77, 773–784 (1994).
Kojima, H., Muto, E., Higuchi, H. & Yanagida, T. Mechanics of single kinesin molecules measured by optical trapping nanometry. Biophys. J. 73, 2012–2022 (1997).
Coppin, C. M., Pierce, D. W., Hsu, L. & Vale, R. D. The load dependence of kinesin's mechanical cycle. Proc. Natl Acad. Sci. USA 94, 8539–8544 (1997).
Meyhöfer, E. & Howard, J. The force generated by a single kinesin molecule against an elastic load. Proc. Natl Acad. Sci. USA 92, 574–578 (1995).
Visscher, K., Gross, S. P. & Block, S. M. Construction of multiple-beam optical traps with nanometer-resolution position sensing. IEEE J. Sel. Topics Quantum. Electron. 2, 1066–1076 (1996).
Svoboda, K. & Block, S. M. Biological applications of optical forces. Annu. Rev. Biophys. Biomol. Struct. 23, 247–285 (1994).
Finer, J. T., Simmons, R. M. & Spudich, J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–119 (1994).
Wang, M. D., Yin, H., Landick, R., Gelles, J. & Block, S. M. Stretching DNA with optical tweezers. Biophys. J. 72, 1335–1346 (1996).
Wang, M. D. et al. Force and velocity measured for single molecules of RNA polymerase. Science 282, 902–907 (1998).
Howard, J. The mechanics of force generation by kinesin. Biophys. J. 68, 245s–255s (1995).
Hackney, D. Kinesin ATPase: Rate-limiting ADP release. Proc. Natl Acad. Sci. USA 85, 6314–6318 (1988).
Gilbert, S. P., Webb, M. R., Brune, M. & Johnson, K. A. Pathway of processive ATP hydrolysis by kinesin. Nature 373, 671–676 (1995).
Ma, Y. Z. & Taylor, E. W. Mechanism of microtubule kinesin ATPase. Biochemistry 34, 13242–13251 (1995).
Svoboda, K., Mitra, P. P. & Block, S. M. Fluctuation analysis of motor protein movement and single enzyme kinetics. Proc. Natl Acad. Sci. USA 91, 11782–11786 (1994).
Schnitzer, M. J. & Block, S. M. Statistical kinetics of processive enzymes. Cold Spring Harbor Symp. Quant. Biol. 60, 793–802 (1995).
Samuel, A. D. T. & Berg, H. C. Torque-generating units of the bacterial flagellar motor step independently. Biophys. J. 71, 918–923 (1996).
Leibler, S. & Huse, D. A. Porters versus rowers: a unified stochastic model of motor proteins. J. Cell Biol. 121, 1357–1368 (1993).
Peskin, C. & Oster, G. Coordinated hydrolysis explains the mechanical behavior of kinesin. Biophys. J. 68, 202s–211s (1995).
Duke, T. & Leibler, S. Motor protein mechanics: a stochastic model with minimal mechanochemical coupling. Biophys. J. 71, 1235–1247 (1996).
Derényi, I. & Vicsek, T. The kinesin walk: a dynamic model with elastically coupled heads. Proc. Natl Acad. Sci. USA 93, 6775–6779 (1996).
Astumian, R. D. Thermodynamics and kinetics of a brownian motor. Science 276, 917–922 (1997).
Higuchi, H. & Yanagida, T. Force generation and detachment of single kinesin molecules activated by laser photolysis of caged ATP and ADP. Cell Struct. Funct. 23, suppl., 198 (1998).
Coppin, C. M., Finer, J. T., Spudich, J. A. & Vale, R. D. Detection of sub-8-nm movements of kinesin by high-resolution optical-trap microscopy. Proc. Natl Acad. Sci. USA 93, 1913–1917 (1996).
Acknowledgements
We thank Y. Inoue, M. Nishiyama, L. Satterwhite and M. Wang for discussions; J. de Georgis for squid dissection; and D. Peoples for machining. This work was supported by grants to S.M.B. from the NIGMS, NSF and W. M. Keck Foundation, predoctoral fellowships to M.J.S. to the American Heart Association, the Charlotte Elizabeth Proctor Fund, and the Program in Mathematics and Molecular Biology Burroughs Wellcome Fund, and a postdoctoral fellowship to K.V. from the Burroughs Wellcome Fund of the Life Sciences Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Visscher, K., Schnitzer, M. & Block, S. Single kinesin molecules studied with a molecular force clamp. Nature 400, 184–189 (1999). https://doi.org/10.1038/22146
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/22146
This article is cited by
-
A Semi-Markov Approach to Study a Group of Kinesin Motors
Bulletin of Mathematical Biology (2024)
-
Artificial microtubules for rapid and collective transport of magnetic microcargoes
Nature Machine Intelligence (2022)
-
Competing instabilities reveal how to rationally design and control active crosslinked gels
Nature Communications (2022)
-
Probing nanomotion of single bacteria with graphene drums
Nature Nanotechnology (2022)
-
A model of processive walking and slipping of kinesin-8 molecular motors
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.