Abstract
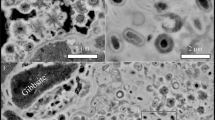
Minerals with mixed valence states are widespread and form in many different rock types1. They can contain, for example, Fe2+–Fe3+ and Mn2+–Mn3+–Mn4+, with the ratios of oxidation states reflecting the redox conditions under which the host materials crystallized. The distribution of the ratio of iron (III) to total iron content (Fe3+/ΣFe) in minerals reflects the oxidation states of their host rocks and is therefore important for answering fundamental questions about the Earth's evolution and structure2,3,4,5,6,7,8. Iron is the most sensitive and abundant indicator of oxidation state, but many mineral samples are too fine-grained and heterogeneous to be studied by standard methods such as Mössbauer spectroscopy, electron microprobe, and wet chemistry. Here we report on the use of electron energy-loss spectroscopy with a transmission electron microscope to determine Fe3+/ΣFe in minerals at the nanometre scale. This procedure is efficient for determining Fe3+/ΣFe ratios of minor and major amounts of iron on a scale heretofore impossible and allows information to be obtained not only from ultra-fine grains but also, for example, at reaction fronts in minerals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Burns, R. G. in Mixed Valency Systems: Applications in Chemistry, Physics and Biology (ed. Prassides, K.) 175–199 (Kluwer Academic, Boston, 1991).
Arculus, R. J. Oxidation status of the mantle: Past and present. Annu. Rev. Earth Planet. Sci. 13, 75–95 (1985).
Carmichael, I. S. E. & Ghiorso, M. S. Oxidation–reduction relations in basic magma: a case for homogenous equilibria. Earth Planet. Sci. Lett. 78, 200–210 (1986).
Wood, B. J. & Virgo, D. Upper mantle oxidation state: Ferric iron contents of lherzolite spinels by 57Fe Mössbauer spectroscopy and resultant oxygen fugacities. Geochim. Cosmochim. Acta 53, 1277–1291 (1989).
Canil, D., Virgo, D. & Scarfe, C. M. Oxidation state of mantle xenoliths from British Columbia, Canada. Contrib. Mineral. Petrol. 104, 453–462 (1990).
Luth, R. W., Virgo, D., Boyd, F. R. & Wood, B. J. Ferric iron in mantle derived garnets: Implications for thermobarometry and for the oxidation state of the mantle. Contrib. Mineral. Petrol. 104, 56–72 (1990).
McCammon, C., Hutchison, M. & Harris, J. Ferric iron content of mineral inclusions in diamonds from Sao Luiz: A view into the lower mantle. Science 278, 434–436 (1997).
Holland, H. D. Model for the evolution of the earth's atmosphere. Geol. Soc. Am. (Budington vol.) 447–477 (1962).
Virgo, D., Luth, R. W., Moats, M. A. & Ulmer, G. C. Constraints on the oxidation state of the mantle: An electrochemical and 57Fe Mössbauer study of mantle-derived ilmenites. Geochim. Cosmochim. Acta 52, 1781–1794 (1988).
Hofer, H. E., Brey, G. P., Schulz-Dobrick, B. & Oberhansli, R. The determination of the oxidation state of iron by the electron microprobe. Eur. J. Mineral. 6, 407–418 (1994).
Delaney, J. S., Bajt, S., Sutton, S. R. & Dyar, M. D. in Mineral Spectroscopy: A Tribute to Roger G. Burns (eds Dyar, M. D., McCammon, C. & Schaefer, M. W.) 165–171 (Spec. Publ. No. 5, Geochemical Soc., 1996).
Droubay, T., Mursky, G. & Tonner, B. P. High-resolution x-ray absorption microspectroscopy of lamellar phases in natural ilmenite. J. Electron. Spectrosc. 84, 159–169 (1997).
Raeburn, S. P., Ilton, E. S. & Veblen, D. R. Quantitative determination of the oxidation state of iron in biotite using X-ray photoelectron spectroscopy: I. Calibration. Geochim. Cosmochim. Acta 61, 4519–4530 (1997).
Raeburn, S. P., Ilton, E. S. & Veblen, D. R. Quantitative determination of the oxidation state of iron in biotite using X-ray photelectron spectroscopy: II. In situ analyses. Geochim. Cosmochim. Acta 61, 4531–4537 (1997).
Harris, J., Hutchison, M. T., Hursthouse, M., Light, M. & Harte, B. Anew tetragonal silicate mineral occurring as inclusions in lower-mantle diamonds. Nature 387, 486–488 (1997).
Mao, H.-K., Shen, G. & Hemley, R. J. Multivariable dependence of Fe-Mg partitioning in the lower mantle. Science 278, 2098–2100 (1997).
Wood, B. J. & Rubie, D. C. The effect of alumina on phase transformations at the 660-kilometer discontinuity from Fe-Mg partitioning experiments. Science 273, 1522–1524 (1996).
Disko, M. M., Ahn, C. C. & Fultz, B. Transmission Electron Energy Loss Spectrometry in Materials Science (Minerals, Metals & Materials Soc., Warrendale, IL, 1992).
Garvie, L. A. J. & Craven, A. J. High-resolution parallel electron energy-loss spectroscopy of Mn L2,3-edges in inorganic manganese compounds. Phys. Chem. Minerals 21, 191–206 (1994).
Garvie, L. A. J. & Buseck, P. R. in Boron Mineralogy, Petrology and Geochemistry (eds Grew, E. S. & Anovitz, L. M.) 821–843 (Reviews in Mineralogy vol. 33, Mineralogical Soc. Am., Washington DC, 1996).
Garvie, L. A. J., Craven, A. J. & Brydson, R. Use of electron-energy loss near-edge fine structure in the study of minerals. Am. Mineral. 79, 411–425 (1994).
Egerton, R. F. Electron Energy-Loss Spectroscopy in the Electron Microscope 2nd edn (Plenum, New York, 1996).
van der Laan, G. & Kirkman, I. W. The 2p absorption spectra of 3d transition metal compounds in tetrahedral and octahedral symmetry. J. Phys.: Condens. Matter. 4, 4189–4204 (1992).
Browning, N. D., Chisholm, M. F. & Pennycook, S. J. Atomic-resolution chemical analysis using a scanning transmission electron microscope. Nature 366, 143–146 (1993).
Bradley, J. P. Nanometer-scale mineralogy and petrography of fine-grained aggregates in anhydrous interplanetary dust particles. Geochim. Cosmochim. Acta 58, 2123–2134 (1994).
Colliex, C. New trends in STEM-based nano-EELS analysis. J. Electron Microsc. 45, 44–50 (1996).
Czank, M., Mayer, J. & Klein, U. Electron spectroscopic imaging (ESI): A new method to reveal the existence of nm-scale exsolution lamellae. Eur. J. Mineral. 9, 1199–1206 (1997).
Righter, K. & Carmichael, I. S. E. Mega-xenocrysts in alkali olivine basalts: Fragments of disrupted assemblages. Am. Mineral. 78, 1230–1245 (1993).
McGuire, A. V., Francis, C. A. & Dyar, M. D. Minerals standards for electron microprobe analysis of oxygen. Am. Mineral. 77, 1087–1091 (1992).
Gudmundsson, G. & Holloway, J. R. Activity–composition relationships in the system Fe-Pt at 1300 and 1400 °C and at 1 atm and 20 kbar. Am. Mineral. 78, 178–186 (1993).
Van Aken, P. A., Liebscher, B. & Styrsa, V. S. Quantitative determination of iron oxidation states in minerals using Fe L2,3-edge electron energy-loss near-edge structure spectroscopy. Phys. Chem. Miner. 5, 323–327 (1998).
Acknowledgements
We thank P. Rez and J. Bradley for discussions, and K. Righter, D. Canil and G.Gudmundsson for providing us with samples. This work was supported by the Earth Sciences Division of the US NSF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garvie, L., Buseck, P. Ratios of ferrous to ferric iron from nanometre-sized areas in minerals. Nature 396, 667–670 (1998). https://doi.org/10.1038/25334
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/25334
This article is cited by
-
MnEdgeNet for accurate decomposition of mixed oxidation states for Mn XAS and EELS L2,3 edges without reference and calibration
Scientific Reports (2023)
-
Ubiquitous and progressively increasing ferric iron content on the lunar surfaces revealed by the Chang’e-5 sample
Nature Astronomy (2023)
-
Impact-driven disproportionation origin of nanophase iron particles in Chang’e-5 lunar soil sample
Nature Astronomy (2022)
-
Advanced characterization of biomineralization at plaque layer and inside rice roots amended with iron- and silica-enhanced biochar
Scientific Reports (2021)
-
PCN-Fe(III)-PTX nanoparticles for MRI guided high efficiency chemo-photodynamic therapy in pancreatic cancer through alleviating tumor hypoxia
Nano Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.