Abstract
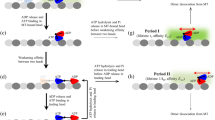
Kinesin is a two-headed, ATP-dependent motor protein1,2 that moves along microtubules indiscrete steps3 of 8 nm. In vitro, single molecules produceprocessive movement4,5, motors typically take ∼100steps before releasing from a microtubule5,6,7 . A central question relates tomechanochemical coupling in this enzyme: how many molecules ofATP are consumed per step? For the actomyosin system,experimental approaches to this issue have generated considerablecontroversy8,9. Here we take advantage of theprocessivity of kinesin to determine the coupling ratio withoutrecourse to direct measurements of ATPase activity, which aresubject to large experimental uncertainties8,10,11,12. Beads carrying singlemolecules of kinesin moving on microtubules were tracked with highspatial and temporal resolution by interferometry3,13. Statistical analysis of theintervals between steps at limiting ATP, and studies offluctuations in motor speed as a function of ATPconcentration14,15, allow the coupling ratio to bedetermined. At near-zero load, kinesin moleculeshydrolyse a single ATP molecule per 8-nm advance. Thisfinding excludes various one-to-many andmany-to-one coupling schemes, analogous to thoseadvanced for myosin, and places severe constraints on models for movement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Vale, R. D., Reese, T. S. & Sheetz, M. P. Identification of a novel force-generating enzyme, kinesin, involved in microtubule-based motility. Cell 42, 39–50 (1985).
Kreis, T. & Vale, R. D. Guidebook to the Cytoskeletal and Motor Proteins(Oxford Univ. Press, (1993)).
Svoboda, K., Schmidt, C. F., Schnapp, B. J. & Block, S. M. Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727 (1993).
Howard, J., Hudspeth, A. J. & Vale, R. D. Movement of microtubules by single kinesin molecules. Nature 342, 154–158 (1989).
Block, S. M., Goldstein, L. S. B. & Schnapp, B. J. Bead movement by single kinesin molecules studied with optical tweezers. Nature 348, 348– 352 (1990).
Vale, R. D. et al. Direct observation of single kinesin molecules moving along microtubules. Nature 380, 451– 453 (1996).
Hackney, D. D. Highly processive microtubule-stimulated ATP hydrolysis by dimeric kinesin head domains. Nature 377, 448– 450 (1995).
Burton, K. Myosin step size: estimates from motility assays and shortening muscle. J. Muscle Res. Cell Motil. 13, 590– 607 (1992).
Yanagida, T., Harada, Y. & Ishijima, A. Nano-manipulation of actomyosin molecular motors in vitro: a new working principle. Trends Biochem. Sci. 18, 319–324 (1993).
Sowerby, A. J., Seehra, C. K., Lee, M. & Bagshaw, C. R. Turnover of fluorescent nucleoside triphosphates by isolated immobilized myosin filaments: transient kinetics on the zeptomole scale. J. Mol. Biol. 234, 114–123 (1993).
Harada, Y., Sakurada, K., Aoki, T., Thomas, D. D. & Yanagida, T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J. Mol. Biol. 216, 49–68 (1990).
Toyoshima, Y. Y., Kron, S. J. & Spudich, J. A. The myosin step size: measurement of the unit displacement per ATP hydrolyzed in an in vitro assay. Proc. Natl Acad. Sci. USA 87, 7130–7134 ( 1990).
Svoboda, K. & Block, S. M. Force and velocity measured for single kinesin molecules. Cell 77, 773– 784 (1994).
Svoboda, K., Mitra, P. P. & Block, S. M. Fluctuation analysis of motor protein movement and single enzyme kinetics. Proc. Natl Acad. Sci. USA 91 , 11782–11786 (1994).
Schnitzer, M. J. & Block, S. M. Statistical kinetics of processive enzymes. Cold Spring Harb. Symp. Quant. Biol. 60, 793–802 (1995).
Hackney, D. D. The rate-limiting step in microtubule-stimulated ATP hydrolysis by dimeric kinesin head domains occurs while bound to the microtubule. J. Biol. Chem. 269, 16508–16511 (1994).
Gilbert, S. P., Webb, M. R., Brune, M. & Johnson, K. A. Pathway of processive ATP hydrolysis by kinesin. Nature 373, 671–676 (1995).
Ma, Y. & Taylor, E. W. Kinetic mechanism of the kinesin motor domain. Biochemistry 34, 13242– 13251 (1995).
Leibler, S. & Huse, D. A. Porters versus rowers: a unified stochastic model of motor proteins. J. Cell Biol. 121 , 1357–1368 (1993).
Coppin, C. M., Finer, J. T., Spudich, J. A. & Vale, R. D. Detection of sub-8-nm movements of kinesin by high-resolution optical-trap microscopy. Proc. Natl Acad. Sci. USA 93, 1913–1917 (1996).
Samuel, A. D. T. & Berg, H. C. Torque-generating units of bacterial flagellar motor step independently. Biophys. J. 71, 918–923 ( 1996).
Block, S. M. & Svoboda, K. Analysis of high resolution recordings of motor movement. Biophys. J. 68, 230s– 241s (1995).
Duke, T. & Leibler, S. Motor protein mechanics: a stochastic model with minimal mechanochemical coupling. Biophys. J. 71, 1235–1247 (1996).
Hackney, D. D. Evidence for alternating head catalysis by kinesin during microtubule-stimulated ATP hydrolysis. Proc. Natl Acad. Sci. USA 91, 6865–6869 (1994).
Gelles, J. et al. Structural and functional features of one- and two-headed biotinated kinesin derivatives. Biophys. J. 68, 276s–282s (1995).
Howard, J. The movement of kinesin along microtubules. Annu. Rev. Physiol. 58, 703–729 ( 1996).
Peskin, C. S. & Oster, G. Coordinated hydrolysis explains the mechanical behavior of kinesin. Biophys. J. 68, 202s–211s (1995).
Derenyi, I. & Vicsek, T. The kinesin walk: a dynamic model with elastically coupled heads. Proc. Natl Acad. Sci. USA 93, 6775–6779 (1996).
Kull, F. J., Sablin, E. P., Lau, R., Fletterick, R. J. & Vale, R. D. Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature 380, 550–555 (1996).
Block, S. M. Fifty ways to love your lever: myosin motors. Cell 87, 151–157 (1996).
Acknowledgements
We thank S. Gross, W. Ryu, L. Satterwhite, M. Wang and especially K. Visscher for technical assistance and discussions; S. Gross for help in purifying kinesin; and P. Mitra and K. Svoboda for advice in the early stages of this project. This work was supported by a grant from NIGMS (S.M.B.) and predoctoral fellowships from NSF and American Heart Association (M.J.S.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schnitzer, M., Block, S. Kinesin hydrolyses one ATP per 8-nm step. Nature 388, 386–390 (1997). https://doi.org/10.1038/41111
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/41111
This article is cited by
-
NMNAT2 supports vesicular glycolysis via NAD homeostasis to fuel fast axonal transport
Molecular Neurodegeneration (2024)
-
Tracking nanoscopic motion with minima of light
Nature Photonics (2023)
-
Kinesin-3 motors are fine-tuned at the molecular level to endow distinct mechanical outputs
BMC Biology (2022)
-
Self-mixing in microtubule-kinesin active fluid from nonuniform to uniform distribution of activity
Nature Communications (2022)
-
Estimating time-dependent entropy production from non-equilibrium trajectories
Communications Physics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.