Abstract
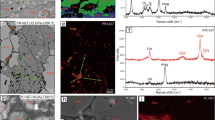
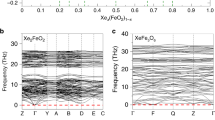
The lower mantle constitutes more than half the Earth's interior by volume, and is believed to consist predominantly of (Mg,Fe)SiO3 perovskite with up to approximately 20% (Mg,Fe)O. In the system FeO–MgO–SiO2, iron partitions preferentially into (Mg,Fe)O relative to the perovskite phase and has been believed to be nearly all in the form of Fe2+on the basis of experiments in the MgO–FeO–SiO2 system1,2,3,4. Here, however, we present a Mössbauer study of (Mg,Fe)SiO3 perovskite containing 3.3 mol% Al2O3, which shows that approximately 50% of the iron is Fe3+. These results, combined with evidence from other experiments, suggest that the proportion of iron present as Fe3+in the lower-mantle perovskite phase is probably much higher than is currently believed. Because the oxidation state of iron in the perovskite phase affects the electrostatic charge balance and equilibrium defect concentration, the presence of Fe3+is likely to significantly affect physical and chemical properties of the lower mantle such as sub- and super-solidus phase relations, transport properties, mechanical behaviour, trace-element partitioning and the concentration of species such as hydroxide ions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ito, E., Takahashi, E. & Matsui, Y. The mineralogy and chemistry of the lower mantle: an implication of the ultrahigh-pressure phase relations in the system MgO-FeO-SiO2. Earth Planet. Sci. Lett. 67, 238–248 (1984).
Guyot, F., Madon, M., Peyronneau, J. & Poirier, J. P. X-ray microanalysis of high-pressure/high-temperature phases synthesized from natural olivine in a diamond anvil cell. Earth Planet. Sci. Lett. 90, 52–64 (1988).
Fei, Y., Mao, H. K. & Mysen, B. O. Experimental determination of element partitioning and calculation of phase relations in the MgO-FeO-SiO2 system at high pressure and high temperature. J. Geophys. Res. 96, 2157–2169 (1991).
Kesson, S. E. & Fitz Gerald, J. D. Partitioning of MgO, FeO, NiO, MnO and Cr2O3 between magnesian silicate perovskite and magnesiowüstite: Implications for the origin of inclusions in diamond and the composition of the lower mantle. Earth Planet. Sci. Lett. 111, 229–240 (1991).
O'Neill, B. & Jeanloz, R. MgSiO3-FeSiO3-Al2O3 in the Earth's lower mantle: Perovskite and garnet at 1,200 km depth. J. Geophys. Res. 99, 19901–19915 (1994).
Keppler, H., McCammon, C. A. & Rubie, D. C. Crystal field and charge transfer spectra of (Mg,Fe)SiO3 perovskite. Am. Mineral. 79, 1215–1218 (1994).
McCammon, C. A., Rubie, D. C., Ross, C. R., Seifert, F. & O'Neill, H. S. C. Mössbauer spectra of 57Fe0.05Mg0.95SiO3 perovskite at 80 and 298 K. Am. Mineral. 77, 894–897 (1992).
Fei, Y., Virgo, D., Mysen, B. O., Wang, Y. & Mao, H. K. Temperature dependent electron delocalization in (Mg,Fe)SiO3 perovskite. Am. Mineral. 79, 826–837 (1994).
McCammon, C. A. The crystal chemistry of ferric iron in Fe0.05Mg0.95SiO3 as determined by Mössbauer spectroscopy. Phys. Chem. Miner.(submitted).
Pownceby, M. I. & O'Neill, H. S. C. Thermodynamic data from redox reactions at high temperatures. IV. Calibration of the Re-ReO2 oxygen buffer from EMF and NiO + Ni-Pd redox sensor measurements. Contrib. Mineral. Petrol. 118, 130–137 (1994).
McCammon, C. A. Crystal chemistry of iron-containing perovskites. Phase Transitions 58, 1–26 (1996).
Kesson, S. E., Fitz Gerald, J. D., Shelley, J. M. G. & Withers, R. L. Phase relations, structure and crystal chemistry of some aluminous silicate perovskites. Earth Planet. Science Lett. 134, 187–201 (1995).
Wood, B. J. & Rubie, D. C. The effect of alumina on phase transformations at the 660-kilometer discontinuity from Fe-Mg partitioning experiments. Science 273, 1522–1524 (1996).
Farges, F., Guyot, F., Andrault, D. & Wang, Y. Local structure around Fe in Mg0.9Fe0.1SiO3 perovskite: An X-ray absorption spectroscopy study at Fe-K edge. Eur. J. Mineral. 6, 303–312 (1994).
Irifune, T. Absence of an aluminous phase in the upper part of the Earth's lower mantle. Nature 370, 131–133 (1994).
McCammon, C. A., Harris, J. W., Harte, B. & Hutchison, M. T. Partitioning of ferric iron between lower mantle phases: Results from natural and synthetic samples. J. Conf. Abstr. 1, 390 (1996).
Speidel, D. H. Phase equilibria in the system MgO-FeO-Fe2O3: The 1300 °C isothermal section and extrapolations to other temperatures. J. Am. Ceram. Soc. 50, 243–248 (1967).
Duba, A. G. & Wanamaker, B. J. DAC measurement of perovskite conductivity and implications for the distribution of mineral phases in the lower mantle. Geophys. Res. Lett. 21, 1643–1646 (1994).
Huebner, J. S., Duba, A. & Wiggins, L. B. Electrical conductivity of pyroxene which contains trivalent cations: Laboratory measurements and the lunar temperature profile. J. Geophys. Res. 84, 4652–4656 (1979).
Poirier, J. P. & Peyronneau, J. in High-Pressure Research: Application to Earth and Planetary Sciences (eds Syono, Y. & Manghnani, M. H.) 77–87 (Terra Scientific/American Geophysical Union, Tokyo/Washington DC, 1992).
Acknowledgements
The manuscript was improved through discussions with A. Duba, B. Harte, M.Hutchison, S. Lauterbach, J. Peyronneau, J.-P. Poirer, F. Seifert and F. Visocekas. The sample of natural orthopyroxene was provided by J. Peyronneau and J.-P. Poirier, and the spectrum of the Al-perovskite phase was recorded at 80 K by S. Lauterbach. Mössbauer experiments were performed using equipment provided by F. Seifert, who was supported by Fonds der Chemischen Industrie (Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McCammon, C. Perovskite as a possible sink for ferric iron in the lower mantle. Nature 387, 694–696 (1997). https://doi.org/10.1038/42685
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/42685
This article is cited by
-
Lattice dynamics, sound velocities, and atomic environments of szomolnokite at high pressure
Physics and Chemistry of Minerals (2023)
-
Magma Ocean, Water, and the Early Atmosphere of Venus
Space Science Reviews (2023)
-
Geochemical models of core–mantle differentiation
Acta Geochimica (2022)
-
Incorporation mechanism of Fe and Al into bridgmanite in a subducting mid-ocean ridge basalt and its crystal chemistry
Scientific Reports (2021)
-
Altered chemistry of oxygen and iron under deep Earth conditions
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.