Abstract
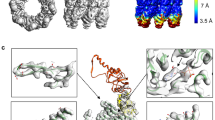
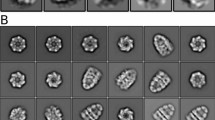
PROTEIN folding mediated by the molecular chaperone GroEL occurs by its binding to non-native polypeptide substrates and is driven by ATP hydrolysis1. Both of these processes are influenced by the reversible association of the co-protein, GroES (refs 2–4). GroEL and other chaperonin 60 molecules5 are large, cylindrical oligomers consisting of two stacked heptameric rings of subunits6,7; each ring forms a cage-like structure8 thought to bind polypeptides in a central cavity8–10. Chaperonins play a passive role in folding by binding or sequestering folding proteins to prevent their aggregation11–13, but they may also actively unfold substrate proteins trapped in misfolded forms, enabling them to assume productive folding conformations14–16. Biochemical studies show that GroES improves the efficiency of GroEL function2,3,17, but the structural basis for this is unknown. Here we report the first direct visualization, by cryo-electron microscopy, of a non-native protein substrate (malate dehydrogenase) bound to the mobile, outer domains at one end of GroEL. Addition of GroES to GroEL in the presence of ATP causes a dramatic hinge opening of about 60°. GroES binds to the equivalent surface of the GroEL outer domains, but on the opposite end of the GroEL oligomer to the protein substrate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hendrick, J. P. & Hartl, F. U. A. Rev. Biochem. 62, 349–384 (1993).
Martin, J., Mayhew, M., Langer, T. & Hartl, F. U. Nature 366, 228–233 (1993).
Fisher, M. J. biol. Chem. 269, 13629–13636 (1994).
Schmidt, M., Buchner, J., Todd, M. J., Lorimer, G. H. & Viitanen, P. V. J. biol. Chem. 267, 10304–10311 (1994).
Kubota, H., Hynes, G., Carne, A., Ashworth, A. & Willison, K. Curr. Biol. 4 89–99 (1994).
Hendrix, R. W. J. molec. Biol. 129, 375–392 (1979).
Hutchinson, E. G., Tichelaar, W., Hofhaus, G., Weiss, H. & Leonard, K. EMBO J. 8, 1485–1490 (1989).
Saibil, H. R. et al. Curr. Biol. 3, 265–273 (1993).
Langer, T., Pfeifer, G., Martin, J., Baumeister, W. & Hartl, F. U. EMBO J. 11, 4757–4765 (1992).
Braig, K., Simon, M., Furuya, F., Hainfeld, J. F. & Horwich, A. L. Proc. natn. Acad. Sci. U.S.A. 90, 3978–3982 (1993).
Ellis, R. J. & Hemmingsen, S. M. Trends biochem. Sci. 14, 339–342 (1989).
Goloubinoff, P., Christeller, J. T., Gatenby, A. A. & Lorimer, G. H. Nature 342, 884–889 (1989).
Nilsson, B. & Anderson, S. A. Rev. Microbiol. 45, 607–635 (1991).
Jackson, G. S. et al. Biochemistry 32, 2554–2563 (1993).
Zahn, R., Spitzfaden, C., Ottiger, M., Wüthrich, K. & Plückthun, A. Nature 368, 261–265 (1994).
Peralta, D., Hartman, D. J., Hoogenraad, N. J. & Høj, P. B. FEBS Lett. 339, 40–45 (1994).
Martin, J. et al. Nature 352, 36–42 (1991).
Mendoza, J. A., Lorimer, G. H. & Horowitz, P. M. J. biol. Chem. 266, 16973–16976 (1991).
Badcoe, I. G. et al. Biochemistry 30, 9195–9200 (1991).
Todd, M. J., Viitanen, P. V. & Lorimer, G. H. Science 265, 659–666 (1994).
Ishii, N., Taguchi, H., Sasabe, H. & Yoshida, M. J. molec. Biol. 236, 691–696 (1994).
Bochkareva, E. S. & Girshovich, A. S. J. biol. Chem. 267, 25672–25675 (1992).
Harris, J. R., Plückthun, A. & Zahn, R. J. struct. Biol. (in the press).
Llorca, O., Marco, S., Carrascosa, J. L. & Valpuesta, J. M. FEBS Lett. 345, 181–186 (1994).
Schmidt, R. et al. Science 265, 656–659 (1994).
Azem, A., Kessel, M. & Goloubinoff, P. Science 265, 653–656 (1994).
Staniforth, R. A. et al. FEBS Lett. 344, 129–135 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, S., Roseman, A., Hunter, A. et al. Location of a folding protein and shape changes in GroEL–GroES complexes imaged by cryo-electron microscopy. Nature 371, 261–264 (1994). https://doi.org/10.1038/371261a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/371261a0
This article is cited by
-
Diversity in heat shock protein families: functional implications in virus infection with a comprehensive insight of their role in the HIV-1 life cycle
Cell Stress and Chaperones (2021)
-
GroEL actively stimulates folding of the endogenous substrate protein PepQ
Nature Communications (2017)
-
Multiple chaperonins in bacteria—novel functions and non-canonical behaviors
Cell Stress and Chaperones (2015)
-
Chaperone-assisted protein folding: the path to discovery from a personal perspective
Nature Medicine (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.