Abstract
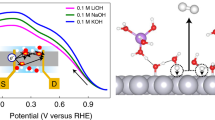
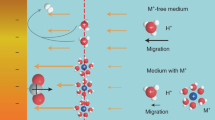
THE rates of many heterogeneous catalytic reactions between gaseous adsorbates on metal surfaces supported on solid electrolytes can be increased by applying a potential to the metal1–8. This phenomenon, which has been reported previously at temperatures of 250–750°C, has been shown9 to be due to the electrochemically induced spillover of ions from the support onto the catalyst surface; the ions then act as promoters for the catalytic reaction. Here we report that a similar effect can be observed in aqueous solution at ambient temperatures. We studied the oxidation of H2 on a platinum/graphite electrode immersed in aqueous KOH. Application of a positive potential of 1–2 V to the Pt electrode increased the rate of H2 oxidation by up to 500%. We deduce that hydroxide ions are acting as promoters, and find that each ion supplied to the catalyst causes the oxidation of up to 20 hydrogen atoms. This kind of rate enhancement for heterogeneous catalytic reactions in solution may be of considerable technological value, for example in the electrochemical treatment of toxic organics10 or the generation of useful industrial chemicals4.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Vayenas, C. G., Bebelis, S. & Neophytides, S. J. phys. Chem. 92, 5083–5085 (1988).
Bebelis, S. & Vayenas, C. G. J. Catal. 118, 125–146 (1989).
Vayenas, C. G., Bebelis, S. & Ladas, S. Nature 343, 625–627 (1990).
Vayenas, C. G., Bebelis, S., Yentekakis, I. V. & Lintz, H.-G. Catal. Today 11, 303–442 (1992).
Marina, O. A. & Sobyanin, V. A. Catal. Lett. 13, 61–70 (1992).
Cavalca, C., Larsen, G., Vayenas, C. G. & Haller, G. L. J. phys. Chem. 97, 6115–6119 (1993).
Yentekakis, I. V., Moggridge, G., Vayenas, C. G. & Lambert, R. M. J. Catal. 146, 292–305 (1994).
Pritchard, J. Nature 343, 592–593 (1990).
Ladas, S., Kennou, S., Bebelis, S. & Vayenas, C. G. J. phys. Chem. 97, 8845–8848 (1993).
Plattner, E. & Comninellis, Ch. in Process Technologies for Water Treatment (ed. Stucki, S.) 205–217 (Plenum, New York, 1988).
Conway, B. E. in Electrodes of Conductive Metallic Oxides (ed. Trasatti, S.) Ch. 9 (Elsevier, Amsterdam, 1981).
Bockris, J. O'M. & Reddy, A. K. N. in Modern Electrochemistry (Plenum, New York, 1973).
Bockris, J. O'M. & Khan, S. U. M. in Surface Electrochemistry, a Molecular Level Approach Ch. 3 (Plenum, New York, 1993).
Conway, B. E. & Tilak, B. V. Adv. Catal. 38, 1–123 (1992).
Chang, S.-C., Leung, L.-W. & Weaver, M. J. J. phys. Chem. 93, 5341–5345 (1989).
Xu, Z., Yates, J. T. Jr, Wang, L. C. & Kreuzer, H. J. J. chem. Phys. 96, 1628–1635 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Neophytides, S., Tsiplakides, D., Stonehart, P. et al. Electrochemical enhancement of a catalytic reaction in aqueous solution. Nature 370, 45–47 (1994). https://doi.org/10.1038/370045a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/370045a0
This article is cited by
-
Electron Donation–Backdonation and the Rules of Catalytic Promotion
Topics in Catalysis (2014)
-
Promotion, Electrochemical Promotion and Metal–Support Interactions: Their Common Features
Catalysis Letters (2013)
-
Baroelectrocatalytic effect: an existence prognosis
Research on Chemical Intermediates (2012)
-
Study of the Mechanism of the Electrochemical Promotion of Rh/YSZ Catalysts for C2H4 Oxidation Via AC Impedance Spectroscopy
Topics in Catalysis (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.