Abstract
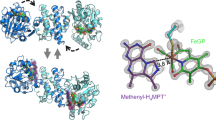
The 2.2 Å crystal structure of the 251K α2β2γ2 dimeric hydroxylase protein of methane mono-oxygenase from Methylococcus capsulatus (Bath) reveals the geometry of the catalytic di-iron core. The two iron atoms are bridged by exogenous hydroxide and acetate ligands and further coordinated by four glutamate residues, two histidine residues and a water molecule. The dinuclear iron centre lies in a hydrophobic active-site cavity for binding methane. An extended canyon runs between αβ pairs, which have many long α-helices, for possible docking of the reductase and coupling proteins required for catalysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Higgins, I. J., Best, D. J. & Hammond, R. C. Nature 286, 561–564 (1980).
Pearman, G. I. & Fraser, P. J. Nature 332, 489–190 (1988).
Colby, J. & Dalton, H. Biochem. J. 171, 461–468 (1978).
Woodland, M. P., Patil, D. S., Cammack, R. & Dalton, H. Biochim, biophys. Acta 873, 237–242 (1986).
Fox, B. G., Surerus, K. K., Münck, E. & Lipscomb, J. D. J. biol. Chem. 263, 10553–10556 (1988).
DeWitt, J. G. et al. J. Am. chem. Soc. 113, 9219–9235 (1991).
Que, L. Jr & True, A. E. Prog. inorg. Chem. 38, 97–200 (1990).
Vincent, J. B., Olivier-Lilley, G. L. & Averill, B. A. Chem. Rev. 90, 1447–1467 (1990).
Kurtz, D. M. Jr & Pickril, B. C. Biochem. biophys. Res. Commun. 181, 337–341 (1991).
Fox, B. G., Shanklin, J., Somerville, C. & Münck, E. Proc. natn. Acad. Sci. U.S.A. 90, 2486–2490 (1993).
Lund, J. & Dalton, H. Eur. J. Biochem. 147, 291–296 (1985).
Green, J. & Dalton, H. J. biol. Chem. 260, 15795–15801 (1985).
Colby, J., Stirling, D. I. & Dalton, H. Biochem. J. 165, 395–402 (1977).
Lindstrom, J. E. et al. Appl. environ. Microbiol. 57, 2514–2522 (1991).
Pritchard, P. H. & Costa, C. F. Environ. Sci. Technol. 25, 372–379 (1991).
Green, J. & Dalton, H. J. biol. Chem. 264, 17698–17703 (1989).
Fox, B. G., Borneman, J. G., Wackett, L. P. & Lipscomb, J. D. Biochemistry 29, 6419–6427 (1990).
Periana, R. A. et al. Science 259, 340–343 (1993).
Ericson, A. et al. J. Am. chem. Soc. 110, 2330–2332 (1988).
DeRose, V., Liu, K. E., Lippard, S. J. & Hoffman, B. J. Am. chem. Soc. 115, 6440–6441 (1993).
Fox, B. G. et al. J. Am. chem. Soc. 115, 3688–3701 (1993).
Thomann, H. et al. J. Am. chem. Soc. 115, 8881–8882 (1993).
Stainthorpe, A. C., Lees, V., Salmond, G. P. C., Dalton, H. & Murrell, J. C. Gene 91, 27–34 (1990).
Nordlund, P., Dalton, H. & Eklund, H. FEBS Lett. 307, 257–262 (1992).
Nordlund, P., Sjöberg, B.-M. & Eklund, H. Nature 345, 593–598 (1990).
Nordlund, P. & Eklund, H. J. molec. Biol. 232, 123–164 (1993).
Prior, S. D. & Dalton, H. FEMS Microbiol. Lett. 29, 105–109 (1985).
Fox, B. G., Froland, W. A., Dege, J. E. & Lipscomb, J. D. J. biol. Chem. 264, 10023–10033 (1989).
Atta, M., Fontecave, M., Wilkins, P. C. & Dalton, H. Eur. J. Biochem. 217, 217–223 (1993).
Hamman, S. et al. Biochem. biophys. Res. Commun. 195, 594–599 (1993).
Andersson, K. K., Elgren, T. E., Que, L. Jr & Lipscomb, J. D. J. Am. chem. Soc. 114, 8711–8713 (1992).
Priestley, N. D. et al. J. Am. chem. Soc. 114, 7561–7562 (1992).
Liu, K. E., Johnson, C. C., Newcomb, M. & Lippard, S. J. J. Am. chem. Soc. 115, 939–947 (1993).
Rardin, R. L., Tolman, W. B. & Lippard, S. J. New. J. Chem. 15, 417–430 (1991).
Poulos, T. L., Finzel, B. C. & Howard, A. J. J. molec. Biol. 195, 687–700 (1987).
Raag, R., Martinis, S. A., Sligar, S. G. & Poulos, T. L. Biochemistry 30, 11420–11429 (1991).
Poulos, T. L., Finzel, B. C., Gunsalus, I. C., Wagner, G. C. & Kraut, J. J. biol. Chem. 260, 16122–16130 (1985).
Liu, K. E. & Lippard, S. J. J. biol. Chem. 266, 12836–12839 (1991).
Fox, B. G., Liu, Y., Dege, J. E. & Lipscomb, J. D. J. biol. Chem. 266, 540–550 (1991).
Froland, W. A., Andersson, K. K., Lee, S.-K., Liu, Y. & Lipscomb, J. D. J. biol. Chem. 267, 17588–17597 (1992).
Liu, K. E., Feig, A. L., Goldberg, D. P., Watton, S. P. & Lippard, S. J. in The Activation of Dioxygen and Homogeneous Catalytic Oxidation (eds Barton, D. H. R., Martell, A. E. & Sawyer, D.) (Plenum, New York, 1993).
Jiang, Y., Wilkins, P. C. & Dalton, H. Biochem. biophys. Acta 1163, 105–112 (1993).
Nordlund, I., Powlowski, J. & Shingler, V. J. Sact. 172, 6826–6833 (1990).
Yen, K. M. et al. J. Bact. 173, 5315–5327 (1991).
Rosenzweig, A. C., Frederick, C. A. & Lippard, S. J. J. molec. Biol. 227, 283–285 (1992).
Knight, S. thesis, Swedish Univ. Agric. Sci., 1989.
Terwillinger, T. C. & Eisenberg, D. Acta crystallogr. A39, 813–817 (1983).
Otwinowski, Z. MLPHARE, CCP4 Proc. 80–88 (Daresbury Lab., Warrington, UK, 1991).
Wang, B. C. Meth. Enzym. 115, 90–112 (1985).
Jones, T. A. in Molecular Replacements (eds Dodson, E.J.) 91–105 (SERC, Daresbury, UK, 1992).
Rould, M. A., Perona, J. J. & Steitz, T. A. Acta crystallogr. A48, 751–756 (1992).
Nordlund, P. thesis, Swedish Univ. Agric. Sci., 1990.
Jones, T. A. & Thirup, S. EMBO J. 5, 829–822 (1985).
Jones, T. A., Bergdoll, M. & Kjeldgaard, M. in Crystallographic Computing and Modeling Methods in Molecular Design (eds Bugg, C. & Ealick, S.) (Springer, New York, 1989).
Stainthorpe, A. C., Murrell, J. C., Salmond, G. P. C., Dalton, H. & Lees, V. Arch. Microbiol. 152, 154–159 (1989).
Brünger, T. A., Kuriyan, J. & Karplus, M. Science 235, 458–460 (1987).
Kraulis, P. J. appl. Crystallogr. 24, 946–950 (1991).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rosenzweig, A., Frederick, C., Lippard, S. et al. Crystal structure of a bacterial non-haem iron hydroxylase that catalyses the biological oxidation of methane. Nature 366, 537–543 (1993). https://doi.org/10.1038/366537a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/366537a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.