Abstract
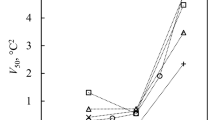
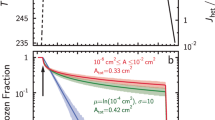
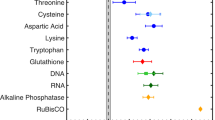
WHEN a dilute ionic solution freezes, differences in the partitioning of ions in the aqueous and ice phases can generate electric potentials which may influence electrochemical reactions1–3. Fitter and co-workers4,5 have studied the influence of freezing on the endothermic oxidation of sulphide to sulphate in growing ice crystals inside a cloud chamber. Here we report that the oxidation of nitrite by dissolved oxygen to form nitrate, which is a very slow process in solution, is accelerated markedly when it takes place in a solution undergoing freezing. At pH 4.5 and a temperature of 25 °C, the rate is increased by a factor of about 105 for a freezing rate of 0.2 g solution per minute; the reaction rate increases as the freezing rate increases. Although the mechanism of this acceleration is not yet clear, we are able to eliminate the possibilities of thennochemical, photochemical and simple electrochemical reactions, and of catalysis on the ice surface. Processes of this sort may be important for chemical reactions taking place in freezing cloud and fog droplets in the atmosphere.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Workman, E. J. & Reynolds, S. E. Phys. Rev. 78, 254–259 (1950).
Lodge, J. P., Baker, M. L. & Pierrard, J. M. J. chem. Phys. 24, 716–719 (1956).
Cobb, A. W. & Gross, G. W. J. electrochem. Soc. 116, 796–804 (1969).
Pitter, R. L., Finnegan, W. G. & Young, L. G. Proc. int. Conf. Global and Regional Environmental Atmospheric Chemistry (eds Newman, L., Wang, W. & Kiang, C. S.) 466–469 (U.S. Department of Energy, Washington DC, 1989).
Finnegan, W. G., Pitter, R. L. & Young, L. G. Atmos. Envir. A25, 2531–2534 (1991).
Maeda, Y. & Munemori, M. Chem. Express. 1, 467–470 (1986).
Damschen, D. E. & Martin, L. B. Atmos. Envir. 17, 2005–2011 (1983).
Nair, S. K. & Peters, L. K. Atmos. Envir. 23, 1399–1423 (1989).
Schwartz, S. E. SO2, NO and NO2 Oxidation Mechanisms: Atmospheric Considerations (ed. Calvert, J. G.) 173–208 (Ann Arbor Science, Boston, 1984).
Hegg, D. A. & Hobbs, P. V. J. atmos. Chem. 7, 325–333 (1988).
Hara, H. J. Japan Soc. Air Pollut. 25, 1–29 (1990).
Platt, U., Perner, D., Harris, G. W., Winer, A. M. & Pitts, J. N. Jr Nature 285, 312–314 (1980).
Harris, G. W. et al. Envir. Sci. Technol. 16, 414–419 (1982).
Sjodin, A. Envir. Sci. Technol. 22, 1086–1089 (1988).
Ruprecht, H. & Sigg, L. Atmos. Envir. A24, 573–584 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takenaka, N., Ueda, A. & Maeda, Y. Acceleration of the rate of nitrite oxidation by freezing in aqueous solution. Nature 358, 736–738 (1992). https://doi.org/10.1038/358736a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/358736a0
This article is cited by
-
Icy reactors break down microplastics
Nature Reviews Materials (2022)
-
Unexpected bias of freeze-drying on the performance assessment of chemical oxidation of soils contaminated by polychlorinated biphenyls
Environmental Chemistry Letters (2019)
-
Effects of oxalic acid on Cr(VI) reduction by phenols in ice
Environmental Science and Pollution Research (2019)
-
Effect of Preservation Techniques on the Determination of Nitrite in Freshwater Samples
Water, Air, and Soil Pollution (2008)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.