Abstract
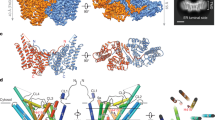
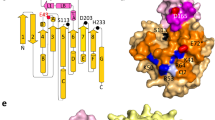
PANCREATIC lipase (triacylglycerol acyl hydrolase) fulfills a key function in dietary fat absorption by hydrolysing triglycerides into diglycerides and subsequently into monoglycerides and free fatty acids. We have determined the three-dimensional structure of the human enzyme, a single-chain glycoprotein of 449 amino acids, by X-ray crystallography and established its primary structure by sequencing complementary DNA clones. Enzymatic activity is lost after chemical modification of Ser 152 in the porcine enzyme1,2, indicating that this residue is essential in catalysis, but other data3,4 are more consistent with a function in interfacial recogni-tion. Our structural results are evidence that Ser 152 is the nucleophilic residue essential for catalysis. It is located in the larger N-terminal domain at the C-terminal edge of a doubly wound parallel β-sheet and is part of an Asp-His-Ser triad, which is chemically analogous to, but structurally different from, that in the serine proteases. This putative hydrolytic site is covered by a surface loop and is therefore inaccessible to solvent. Interfacial activation, a characteristic property of lipolytic enzymes acting on water-insoluble substrates at water-lipid interfaces, probably involves a reorientation of this flap, not only in pancreatic lipases but also in the homologous hepatic and lipoprotein lipases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Maylié, M. F., Charles, M. & Desnuelle, P. Biochim. biophys. Acta 276, 162–175 (1972).
Guidoni, A., Benkouka, F., De Caro, J. & Rovery, M. Biochim. biophys. Acta 660, 148–150 (1981).
Chapus, C. & Sémériva, M. Biochemistry 15, 4988–4991 (1976).
Chapus, C., Sémériva, M., Bovier-Lapierre, C. & Desnuelle, P. Biochemistry 15, 4980–4987 (1976).
De Caro, J. et al. Biochim. biophys. Acta 671, 129–138 (1981).
Kerfélec, B., LaForge, K. S., Puigserver, A. & Scheele, G. Pancreas 1, 430–437 (1986).
Sternby, B. & Borgström, B. Comp. biochem. Physiol. 688, 15–18 (1981).
Bricogne, G. Acta crystallogr. A32, 832–847 (1976).
Jones, T. A. J. appl. Crystallogr. 11, 268–272 (1978).
Brünger, A. T., Kuriyan, J. & Karplus, M. Science 235, 458–460 (1987).
Richardson, J. S. Meth. Enzym. 115, 341–358 (1985).
Richardson, J. S. Proc. natn. Acad. Sci. U.S.A. 73, 2619–2623 (1976).
Sternberg, M. J. E. & Thornton, J. M. J. molec. Biol. 110, 269–283 (1977).
Chapus, C., Rovery, M., Sarda, L. & Verger, R. Biochimie 70, 1223–1234 (1988).
Datta, S. et al. J. biol. Chem. 263, 1107–1110 (1988).
Rotanova, T. V., Klaus, R., Ivanova, A. G., Ginodman, L. & Antonov, V. K. Bioorg. Khim. 2, 837–845 (1976).
Garner, C. W. J. biol. Chem. 255, 5064–5068 (1980).
De Caro, J. D., Rouimi, P. & Rovery, M. Eur. J. Biochem. 158, 601–607 (1986).
De Caro, J. D., Chautan, M. P., Rouimi, P. & Rovery, M. 70, 1785–1790 (1988).
Bengtsson-Olivecrona, G., Olivecrona, T. & Jönvall, H. Eur. J. Biochem. 161, 281–288 (1986).
Bengtsson, G. & Olivecrona, T. Eur. J. Biochem. 113, 547–554 (1981).
Persson, B., Bengtsson-Olivecrona, G., Enerbäck, S., Olivecrona, T. & Jörnvall, H. Eur. J. Biochem. 179, 39–45 (1989).
Kabsch, W. J. appl. Crystallogr. 21, 916–924 (1988).
Dickerson, R. E., Weinzierl, J. E. & Palmer, R. A. Acta crystallogr. B24, 997–1001 (1968).
Kabsch, W. & Sander, C. Biopolymers 22, 2577–2637 (1983).
Bode, W. & Schwager, P. J. molec. Biol. 98, 693–717 (1975).
Bernstein, F. C. et al. J. molec. Biol. 112, 535–543 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Winkler, F., D'Arcy, A. & Hunziker, W. Structure of human pancreatic lipase. Nature 343, 771–774 (1990). https://doi.org/10.1038/343771a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/343771a0
This article is cited by
-
Molecular structures, chemical descriptors, and pancreatic lipase (1LPB) inhibition by natural products: a DFT investigation and molecular docking prediction
Structural Chemistry (2024)
-
Phytochemical analysis, in vitro and in silico effects from Alstonia boonei De Wild stem bark on selected digestive enzymes and adipogenesis in 3T3-L1 preadipocytes
BMC Complementary Medicine and Therapies (2023)
-
Structure of dimeric lipoprotein lipase reveals a pore adjacent to the active site
Nature Communications (2023)
-
Copper Phthalocyanine Improving Nonaqueous Catalysis of Pseudomonas cepacia Lipase for Ester Synthesis
Applied Biochemistry and Biotechnology (2023)
-
In vitro enzyme inhibitory effects of green and brown Australian seaweeds and potential impact on metabolic syndrome
Journal of Applied Phycology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.