Abstract
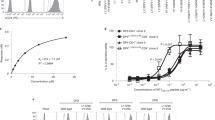
T CELLS recognize protein antigens as fragments (peptides) held in a defined binding site1of class I or class II major histocompatibility (MHC) molecules. The formation of complexes between various immunologically active peptides and different MHC molecules has been demonstrated directly in binding studies between the peptides and solubilized, purified molecules of class II MHC2–6. Studies with intact cells, living or fixed, have not directly demonstrated the binding of the peptides to MHC molecules on antigen-presenting cells, but the formation of such complexes has been shown indirectly through the capacity of antigen-presenting cells to stimulate specific T cells7–9. Here we report evidence that supports directly the binding of radiolabelled influenza matrix peptide 17–29 to products of the human class II MHC locus HLA-DR, on living homozygous B-cell lines, and we show that the kinetics of such binding is much faster with living cells than with fixed cells. Furthermore, whereas the peptide reacts with HLA-DR molecules of all alleles, it binds preferentially to DR1, the restricting element in antigen presentation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bjorkman, P. et al. Nature 329, 512–519 (1987).
Babbit, B. P., Allen, P. M., Matsueda, G., Haber, E. & Unanue, E. R. Nature 317, 359–361 (1985).
Buus, S., Sette, A., Colon, S. M., Jevis, D. M. & Grey, H. M. Cell 47, 1071–1077 (1986).
Guillet, J. G., Lai, M. Z., Briner, T. J., Smith, J. A. & Gefter, M. L. Nature 324, 260–262 (1987).
Watts, T. H. & McConnell, H. M. Proc. natn. Acad. Sci. U.S.A. 83, 9660–9664 (1986).
Luescher, I. F., Allen, P. M. & Unanue, E. R. Proc. natn. Acad. Sci. U.S.A. 85, 871–874 (1988).
Shimonkevitz, R., Kappler, J., Marrack, P. & Grey, H. J. exp. Med. 158, 303–316 (1983).
Shimonkevitz, R., Colon, S., Kappler, J. W., Marrack, P. & Grey, H. M. J. Immun. 133, 2067–2074 (1984).
Roosneck, E., Demotz, S., Corradin, G. & Lanzavecchia, A. J. Immun. 140, 4079–4082 (1988).
Eckels, D., et al. Immunogenetics 19, 409–423 (1984).
Rothbard, J. B. et al. Cell 52, 515–523 (1988).
Pernis, B. Immunol. Today 6, 45–49 (1985).
Harding, C. V. & Unanue, E. R. J. Immun. 142, 12–19 (1989).
Limbird, L. Cell Surface Receptors (Nijhoff, Boston, 1986).
Nairn, R., Spengler, M. L., Hoffman, M. D., Solvay, M. J. & Thomas, D. W. J. Immun. 133, 3225–3234 (1984).
Lakey, E. K., Margoliash, E. & Pierce, S. K. Proc. natn. Acad. Sci. U.S.A. 84, 1659–1663 (1987).
Trucco, M., de Petris, S., Garotta, G. & Ceppellini, R. Human Immunol. 3, 233–243 (1980).
Buus, S., Sette, A., Colon, S. M. & Grey, H. M. Science 242, 1045–1047 (1988).
Merrified, R. B. J. Am. chem. Soc. 85, 2149–2154 (1963).
Yajima, H. et al. Chem. Pharm. Bull 23, 371–374 (1975).
Greenwood, F. C., Hunter, W. M. & Glover, J. S. Biochem. J. 89, 114–123 (1963).
Trucco, M. M., Garotta, G., Stocker, J. W. & Ceppellini, R. Immunol. Rev. 47, 237–242 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ceppellini, R., Frumento, G., Ferrara, G. et al. Binding of labelled influenza matrix peptide to HLA DR in living B lymphoid cells. Nature 339, 392–394 (1989). https://doi.org/10.1038/339392a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/339392a0
This article is cited by
-
Normal HBsAg presentation and T-cell defect in the immune response of nonresponders
Immunogenetics (1995)
-
Peptide binding to class I MHC on living cells and quantitation of complexes required for CTL lysis
Nature (1991)
-
Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments
Nature (1990)
-
HLA-linkedImmune suppression genes
Japanese journal of human genetics (1990)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.