Abstract
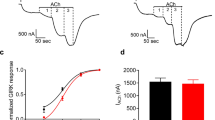
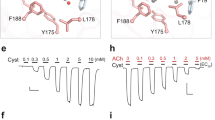
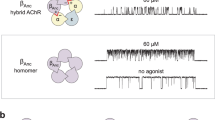
The muscarinic acetylcholine receptor (mAChR) mediates various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels, through the action of guanine nucleotide-binding regulatory proteins1–3. Pharmacologically distinguishable forms of the mAChR occur in different tissues and have provisionally been classified into M1 (I), M2 cardiac (II) and M2 glandular (III) subtypes on the basis of their difference in apparent affinity for antagonists4–8. In an attempt to elucidate the molecular basis of the functional heterogeneity of the mAChR, we have cloned and sequenced DNAs complementary to porcine cerebral and cardiac messenger RNAs encoding mAChRs and have thereby deduced the primary structures of the receptor proteins9,10. We report here that the messenger RNA generated by transcription of the cardiac complementary DNA directs the formation of a functional mAChR in Xenopus oocytes and that this mAChR differs from the mAChR formed by expression of the cerebral cDNA9 both in acetylcholine (ACh)-induced response and in antagonist binding properties. Our results provide evidence indicating that the mAChR encoded by the cerebral cDNA (designated as mAChR I) and the mAChR encoded by the cardiac cDNA (mAChR II) are of the M1 (I) and the M2 cardiac (II) subtype, respectively.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
1. Burgen, A. S. V. Trends pharmac. Sci. Suppi 5, 1–3 (1984). 2. Brown, D. Nature 319, 358–359 (1986). 3. Oilman, A. G. Trends Neurosci. 9, 460–463 (1986). 4. Hammer, R., Berrie, C. P., Birdsall, N. J. M., Burgen, A. S. V. & Hulme, E. C. Nature 283, 90–92 (1980). 5. Birdsall, N. J. M. & Hulme, E. C. Trends pharmac. Sci. 4, 459–463 (1983). 6. Hammer, R., Giraldo, E., Schiavi, G. B., Monferini, E. & Ladinsky, H. Life Sci. 38,1653–1662 (1986). 7. Watson, M. et al. Trends pharmac. Sci. Suppl. 7, 46–55 (1986). 8. Birdsall, N. J. M. et al. Biochem. Soc. Symp. 52, 23–32 (1986). 9. Kubo, T. et al. Nature 323, 411–416 (1986). 10. Kubo, T. et al. FEBS Lett. 209, 367–372 (1986). 11. Melton, D. A. et al. Nucleic Acids Res. 12, 7035–7056 (1984). 12. Barish, M. E. / Physiol., Land. 342, 309–325 (1983). 13. Miledi, R. & Parker, I. /. Physiol., Lond. 357, 173–183 (1984). 14. Dascal, N., Gillo, B. & Lass, Y. /. Physiol., Lond. 366, 299–313 (1985). 15. Kusano, K., Miledi, R. & Stinnakre, J. / Physiol., Lond. 328, 143–170 (1982). 16. Dascal, N., Landau, E. M. & Lass, Y. / Physiol, Lond. 352, 551–574 (1984). 17. Berrie, C. P., Birdsall, N. J. M., Burgen, A. S. V. & Hulme, E. C. Br. J. Pharmac. Suppl. 78, P67 (1983). 18. Watson, M., Roeske, W. R. & Yamamura, H. I. /. Pharmac. exp. Ther. 237, 419–428 (1986). 19. Hammer, R., Giachetti, A. Life Sci. 31, 2991–2998 (1982). 20. Schimerlik, M. I., Miller, S., Peterson, G. L., Rosenbaum, L. C. & Tota, M. R. Trends pharmac. Sci. Suppl. 7, 2–7 (1986). 21. Cheng, Y.–C. & Prusoff, W. H. Biochem. Pharmac. 22, 3099–3108 (1973). 22. Sugiyama, H., Hisanaga, Y. & Hirono, C. Brain Res. 338, 346–350 (1985). 23. Konarska, M. M., Padgett, R. A. & Sharp, P. A. Cell 38, 731–736 (1984). 24. Sakmann, B. et al. Nature 318, 538–543 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fukuda, K., Kubo, T., Akiba, I. et al. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature 327, 623–625 (1987). https://doi.org/10.1038/327623a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/327623a0
This article is cited by
-
Molecular mechanism of muscarinic acetylcholine receptor M3 interaction with Gq
Communications Biology (2024)
-
Acetylcholine
British Journal of Pharmacology (2006)
-
M1 muscarinic receptors block caspase activation by phosphoinositide 3-kinase- and MAPK/ERK-independent pathways
Cell Death & Differentiation (2000)
-
Pharmacological aspects of acid secretion
Digestive Diseases and Sciences (1995)
-
Inositol 1,4,5-trisphosphate formation and ryanodine-sensitive oscillations of cytosolic free Ca2+ concentrations in neuroblastoma�fibroblast hybrid NL308 cells expressing m2 and m4 muscarinic acetylcholine receptor subtypes
Pfl�gers Archiv European Journal of Physiology (1995)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.