Abstract
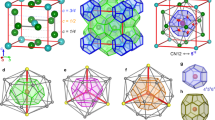
Clathrate hydrates, ice-like host–guest systems containing guest molecules in cages of hydrogen-bonded water molecules exist in three well-characterized cubic forms, and a less well-characterized tetragonal form1,2. On the basis of 2H and l29Xe NMR measurements and X-ray and neutron powder diffraction results, we now report a new hexagonal hydrate structure requiring both large and small guest molecules to stabilize the structure. This hydrate is expected to be isostructural with the hexagonal clathrasil dodecasil-lH (see ref. 14 for clathrasil nomenclature). As for the cubic clathrate hydrates, the new hydrate structure may occur naturally.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Davidson, D. W. in Water. A Comprehensive Treatise Vol. 2 (ed. Franks, F.) 115–234 (Plenum, New York, 1972).
Jeffrey, G. A. in Inclusion Compounds Vol. 1 (eds Atwood, J. L., Davies J. E. D. & MacNicol, D. D.) 135–140 (Academic, London, 1984).
McMullan, R. K. & Jeffrey, G. A. J. chem. Phys. 42, 2725–2731 (1965).
Mak, T. C. W. & McMullan, R. K. J. chem. Phys. 42, 2732–2738 (1965).
Gies, H., Liebau, F. & Gerke, H. Angew. Chem. int. Edn. Engl. 21, 206–207 (1982).
Gies, H. Z. Kristallogr. Kristallgeom. 164, 247–257 (1983); Z. Kristallogr. Kristallgeom. 167, 73–82 (1984).
Gerke, H. & Gies, H. Z. Kristallogr. Kristallgeom. 166, 11–22 (1984).
Gies, H. Fortschr. Miner. 63, 74 (1985).
Davidson, D. W., Ratcliffe, C. I. & Ripmeester, J. A. J. Inclusion Phenomena 2, 239–247 (1985).
Ripmeester, J. A. & Davidson, D. W. J. molec. Struct. 75, 67–72 (1981).
Ripmeester, J. A. J. Am. chem. Soc. 104, 289–290 (1982).
Davidson, D. W. & Ripmeester, J. A. in Inclusion Compounds Vol. 3 (eds Atwood, J. L., Davies, J. E. D. & MacNicol, D. D.) 69–128 (Academic, London, 1984).
Davidson, D. W. et al. Geochim. cosmochim. Acta 50, 619–623 (1986).
Liebau, F., Gies, H., Gunawardane, R. P. & Marler, B. Zeolites 6, 373 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ripmeester, J., Tse, J., Ratcliffe, C. et al. A new clathrate hydrate structure. Nature 325, 135–136 (1987). https://doi.org/10.1038/325135a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/325135a0
This article is cited by
-
Significance of the high-pressure properties and structural evolution of gas hydrates for inferring the interior of icy bodies
Progress in Earth and Planetary Science (2023)
-
Topological dual and extended relations between networks of clathrate hydrates and Frank-Kasper phases
Nature Communications (2023)
-
Spatial distribution and inventory of natural gas hydrate in the Qiongdongnan Basin, northern South China Sea
Journal of Oceanology and Limnology (2023)
-
Gas hydrates in nature and in the laboratory: necessary requirements for formation and properties of the resulting hydrate phase
ChemTexts (2022)
-
Kinetic analysis of arginine, glycine and valine on methane (95%)–propane (5%) hydrate formation
Reaction Kinetics, Mechanisms and Catalysis (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.