Abstract
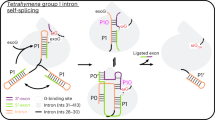
Group I introns include many mitochondrial ribosomal RNA and messenger RNA introns and the nuclear rRNA introns of Tetrahymena and Physarum1–6. The splicing of precursor RNAs containing these introns is a two-step reaction. Cleavage at the 5′ splice site precedes cleavage at the 3′ splice site, the latter cleavage being coupled with exon ligation7–9. Following the first cleavage, the 5′ exon must somehow be held in place for ligation. We have now tested the reactivity of two self-splicing group I RNAs, the Tetrahymena pre-rRNA and the intron 1 portion of the Neurospora mitochondrial cytochrome b (cob) pre-mRNA, in the inter-molecular exon ligation reaction (splicing in trans) described by Inoue et al.10. The different sequence specificity of the reactions supports the idea that the nucleotides immediately upstream from the 5′ splice site are base-paired to an internal, 5′ exon-binding site, in agreement with RNA structure models proposed by Davies and co-workers2,3 and others4,5,11. The internal binding site is proposed to be involved in the formation of a structure that specifies the 5′ splice site and, following the first step of splicing, to hold the 5′ exon in place for exon ligation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Burke, J. & RajBhandary, U. L. Cell 31, 509–520 (1982).
Davies, R. W., Waring, R. B., Ray, J. A., Brown, T. A. & Scazzocchio, C. Nature 300, 719–724 (1982).
Waring, R. B., Scazzocchio, C., Brown, T. A. & Davies, R. W. J. molec. Biol 167, 595–605 (1983).
Michel, F., Jacquier, A. & Dujon, B. Biochimie 64, 867–881 (1982).
Michel, F. & Dujon, B. EMBO J. 2, 33–38 (1983).
Cech, T. R. et al. Proc. natn. Acad. Sci. U.S.A. 80, 3903–3907 (1983).
Zaug, A. J., Grabowski, P. J. & Cech, T. R. Nature 301, 578–583 (1983).
Cech, T. R. Cell 44, 207–210 (1986).
Inoue, T., Sullivan, F. X. & Cech, T. R. J. molec. biol. 189, 143–165 (1986).
Inoue, T., Sullivan, F. X. & Cech, T. R. Cell 43, 431–437 (1985).
Bos, J. L. et al. Cell 20, 207–214 (1980).
Garriga, G. & Lambowitz, A. M. Cell 39, 631–641 (1984).
Tabak, H. F. Van der Horst, G., Osinga, K. A. & Arnberg, A. C. Cell 39, 623–629 (1984).
Hensgens, L. A. M., Bonen, L., de Haan, M., Van der Horst, G. & Grivell, L. A. Cell 32, 379–389 (1983).
Van der Horst, G. & Tabak, H. F. Cell 40, 759–766 (1985).
Sullivan, F. X. & Cech, T. R. Cell 42, 639–648 (1985).
Helmer-Citterich, M., Morelli, G. & Macino, G. EMBO J. 2, 1235–1242 (1983).
Burke, J. M., Breitenberger, C., Heckman, J. E., Dujon, B. & RajBhandary, U. L. J. biol. Chem. 259, 504–511 (1984).
Bass, B. L. & Cech, T. R. Nature 308, 820–826 (1984).
Price, J. V. & Cech, T. R. Science 228, 719–722 (1985).
McMaster, G. K. & Carmichael, G. G. Proc. natn. Acad. Sci. U.S.A. 74, 4835–4838 (1977).
Donis-Keller, H. Nucleic Acids Res. 7, 179–192 (1979).
Lockard, R. E. et al. Nucleic Acids Res. 5, 37–56 (1978).
Donis-Keller, H. Nucleic Acids Res. 8, 3133–3142 (1980).
Been, M. D. & Cech, T. R. Nucleic Acids Res. 13, 8389–8408 (1985).
Waring, R. B., Towner, P., Minter, S. J. & Davies, R. W. Nature 321, 133–139 (1986).
Been, M. D. & Cech, T. R. (submitted).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garriga, G., Lambowitz, A., Inoue, T. et al. Mechanism of recognition of the 5′ splice site in self-splicing group I introns. Nature 322, 86–89 (1986). https://doi.org/10.1038/322086a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/322086a0
This article is cited by
-
The Tetrahymena ribozyme acts like an RNA restriction endonuclease
Nature (1986)
-
Biochemistry: Clues about RNA Enzymes
Nature (1986)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.