Abstract
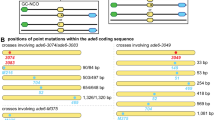
Post-meiotic segregation of alleles, which is seen, for example, in the 5:3 distribution of alleles in the products of a single meiosis in fungi, has been thought to be due to the non-repair of heteroduplex regions formed during genetic recombination. In current models of genetic recombination, heteroduplex DNA is formed either as the primary intermediate generated by two interacting non-sister chromatids1 or as a short region flanking a double-stranded gap2. The frequency of post-meiotic segregation differs for different alleles, and this is presumed to reflect the varying efficiencies with which different types of mismatches in the heteroduplex are repaired. To gain some insight into this process, we have now determined the nucleotide sequences of various yeast alleles with different post-meiotic segregation frequencies and compared the mismatches predicted to occur in heteroduplexes of these alleles with wild-type DNA with those repaired with varying efficiency in bacterial systems. A striking correlation is observed, with the mismatches predicted for high post-meiotic segregation frequency alleles being similar to mismatches repaired with low efficiency in bacteria. These results support the view that post-meiotic segregation frequency reflects heteroduplex repair efficiency and the contention that meiotic gene conversion is the result of the successful repair of heteroduplex mismatches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Meselson, M. S. & Radding, C. M. Proc. natn. Acad. Sci. U.S.A. 72, 358–361 (1975).
Szostak, J. W., Orr-Weaver, T. L., Rothstein, R. J. & Stahl, F. W. Cell 33, 25–35 (1983).
Fogel, S., Choi, T., Kilgore, D., Lusnak, K. & Williamson, M. Recent Adv. Yeast molec. Biol. 1, 269–288 (1982).
Fogel, S. & Choi, T. in Trends in Molecular Genetics (eds Sinha, U. & Klingmüller, W.) 63–80 (Spectrum, Patna, 1985).
Orr-Weaver, T. L., Szostak, J. W. & Rothstein, R. J. Meth. Enzym. 101, 228–245 (1983).
Messing, J. Meth. Enzym. 101, 20–78 (1983).
Esposito, M. S. Genetics 58, 507–527 (1968).
Beacham, I. R., Schweitzer, B. W., Warrick, H. M. & Carbon, J. Gene 29, 271–279 (1984).
Fogel, S., Mortimer, R. K. & Lusnak, K. in Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance (eds Strathern, J. N., Jones, E. W. & Broach, J. R.) 289–339 (Cold Spring Harbor Laboratory, New York, 1981).
Esposito, M. S. Molec. gen. Genet. 111, 297–299 (1971).
Thuriaux, P. et al. Curr. Genet. 1, 89–95 (1980).
De Boer, J. G. & Ripley, L. S. Proc. natn. Acad. Sci. U.S.A. 81, 5528–5531 (1984).
Lacks, S. J. molec. Biol. 5, 119–131 (1962).
Ephrussi-Taylor, H. & Gray, T. C. J. gen. Physiol. 42, 211–231 (1966).
Lacks, S. Genetics 53, 207–235 (1966).
Claverys, J.-P., Méjean, V., Gasc, A.-M. & Sicard, A. M. Proc. natn. Acad. Sci. U.S.A. 80, 5956–5960 (1983).
Kramer, B., Kramer, W. & Fritz, H.-J. Cell 38, 879–887 (1984).
Williamson, M. S. thesis, Univ. California (1984).
Donohue, T. F., Farabaugh, P. J. & Fink, G. R. Gene 18, 47–59 (1982).
Gasc, A.-M. & Sicard, A. M. Genetics 90, 1–18 (1978).
Bennetzen, J. L. & Hall, B. D. J. biol. Chem. 257, 3018–3025 (1982).
Donahue, T. F., Daves, R. S., Lucchini, G. & Fink, G. R. Cell 32, 89–98 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
White, J., Lusnak, K. & Fogel, S. Mismatch-specific post-meiotic segregation frequency in yeast suggests a heteroduplex recombination intermediate. Nature 315, 350–352 (1985). https://doi.org/10.1038/315350a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/315350a0
This article is cited by
-
Genome-wide survey of post-meiotic segregation during yeast recombination
Genome Biology (2011)
-
Polarity of meiotic gene conversion in fungi: Contrasting views
Experientia (1994)
-
Control of the expression of the ADE2 gene of the yeast Saccharomyces cerevisiae
Current Genetics (1994)
-
The yeast gene MSH3 defines a new class of eukaryotic MutS homologues
Molecular and General Genetics MGG (1993)
-
Regulation of the ADE2 gene from Saccharomyces cerevisiae
Current Genetics (1993)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.