Abstract
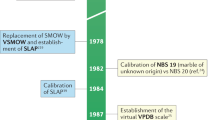
Use of light stable isotope ratio measurements has proliferated in the past decade. The need for procuring additional stable isotope reference materials was recognized at an International Atomic Energy Agency (IAEA) consultants' meeting convened in 19761. This group recommended acquisition of two carbonates, two carbon dioxide samples, a biotite, a sulphate, and other reference materials. We report here on the mass-spectrometric analysis of these and other reference samples in a single laboratory to minimize interlaboratory calibration errors. A result of this work is an improved equation for relating the PDB isotope scale (belemnite from the Peedee Formation of South Carolina adopted in the 1950s as a reference in palaeotemperature studies2) to the V-SMOW (Vienna-Standard Mean Ocean Water) scale1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gonfiantini, R. Nature 271, 534–536 (1978).
Craig, H. Geochim. cosmochim. Acta 12, 133–149 (1957).
Epstein, S. & Mayeda, T. Geochim. cosmochim. Acta 4, 213–224 (1953).
McCrea, J. M. J. chem. Phys. 18, 849–857 (1950).
Stuermer, D. H., Peters, K. E. & Kaplan, I. R. Geochim. cosmochim. Acta 42, 989–997 (1978).
Sofer, Z. Analyt. Chem. 52, 1389–1391 (1980).
Rafter, T. A. N. Z. J. Sci. 10, 493–510 (1967).
Mizutani, Y. Geochem. J. 6, 67–73 (1972).
Nehring, N. L., Bowen, P. A. & Truesdell, A. H. Geothermics 5, 63–66 (1977).
Clayton, R. N. & Mayeda, T. K. Geochim. cosmochim. Acta 27, 43–52 (1963).
Coplen, T. & Kendall, C. Analyt. Chem. 54, 2611–2612 (1982).
Coplen, T. Int. J. Mass Spectrom. Ion Phys. 11, 37–40 (1973).
Mook, W. G. & Grootes, P. M. Int. J. Mass Spectrom. Ion Phys. 12, 273–298 (1973).
Friedman, I. & O'Neil, J. R. in U.S. geol. Surv. Prof. Pap. 440-KK (1977).
Sharma, T. & Clayton, R. N. Geochim. cosmochim. Acta 29, 1347–1353 (1965).
O'Neil, J. R. & Epstein, S. J. geophys. Res. 71, 4955–4961 (1966).
Ito, E. & Clayton, R. N. Geochim. cosmochim. Acta 47 (in the press).
Craig, H. Science 133, 1833–1834 (1961).
Blattner, P. & Hulston, J. R. Geochim. cosmochim. Acta 42, 59–62 (1978).
Coplen, T., Kendall, C. & Barnes, I. L. Geostandards Newslett. 6, 257–258 (1982).
Mook, W. G. Palaeogeogr., Palaeoclimatol., Palaeocol. 9, 245–263 (1971).
Letolle, R. Bull. Soc. fr. Minér. Cristallogr. 94, 449–450 (1971).
Friedman, I., O'Neil, J. & Cebula, G. Geostandards Newslett. 6, 11–12 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Coplen, T., Kendall, C. & Hopple, J. Comparison of stable isotope reference samples. Nature 302, 236–238 (1983). https://doi.org/10.1038/302236a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/302236a0
This article is cited by
-
Life in a Central European warm-temperate to subtropical open forest: Paleoecology of the rhinocerotids from Ulm-Westtangente (Aquitanian, Early Miocene, Germany)
The Science of Nature (2024)
-
Diagenetic evolution and reservoir quality of the Oligocene sandstones in the Baiyun Sag, Pearl River Mouth Basin, South China Sea
Acta Oceanologica Sinica (2024)
-
Origin and tectonic environment of the Longbohe Fe-Cu-(LREE) deposit in the Ailao Shan–Red River shear zone, Southwest China: a Neoproterozoic subduction-related IOCG deposit?
Mineralium Deposita (2024)
-
Turkey domestication and provisioning in the Mesa Verde Region (US Southwest), Pueblo I to Pueblo III (725–1280 CE): C, Sr, and O isotope analyses
Archaeological and Anthropological Sciences (2024)
-
Constraining the tectonic evolution of rifted continental margins by U–Pb calcite dating
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.