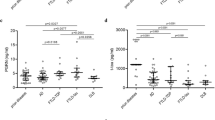
Abstract
Early clinical symptoms of sporadic Creutzfeldt–Jakob disease (CJD) may overlap with other neurodegenerative diseases like Alzheimer's disease (AD) and frontotemporal degeneration (FTD). On entering an era in which pharmaceutical treatment of CJD occurs, reliable diagnostic markers like immunodetection of 14–3–3 proteins in the cerebrospinal fluid (CSF) are required. However, false negative results in autopsy-proven, sporadic CJD cases, as well as false positive results in several other disorders including AD and FTD showing high CSF tau protein levels, limit the potential of this marker. Due to neuronal lysis the cytosolic fraction of total tau containing phosphorylated and non-phosphorylated isoforms is partially liberated into the CSF. Since hyperphosphorylation of tau may specifically occur in neurodegenerative diseases associated with neurofibrillary changes, we hypothesized that the phospho-tau (P-tau)/total tau ratio in CSF may be a useful marker to discriminate CJD from other neurodegenerative disorders. The P-tau/total tau ratio discriminated patients with CJD from all other neuro-degenerative disorders including patients with AD and FTD without any overlap. Although the results have to be confirmed in a larger sample, the preliminary data suggest that simultaneous measurement of total tau and P-tau in CSF may be useful to identify patients with CJD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hsich G, Kenney K, Gibbs C, Lee KH, Harrington MG . The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongiform encephalopathies. N Engl J Med 1996; 335: 924–930.
Zerr I, Pocchiari M, Collins S, Brandel JP, de Pedro Cuesta J, Knight RSG et al. Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology 2000; 55: 811–815.
Burkhard P, Sanchez J-C, Landis T, Hochstrasser DF . CSF detection of the 14-3-3 protein in unselected patients with dementia. Neurology 2001; 56: 1528–1533.
Iqbal K, Grundke-Iqbal I . Mechanism of Alzheimer neurofibrillary degeneration and the formation of tangles. Mol Psychiatry 1997; 2: 178–180.
Otto M, Wiltfang J, Tumani H, Zerr I, Lantsch M, Kornhuber J et al. Elevated levels of Tau protein in CSF of patients with Creutzfeldt–Jakob disease. Neurosci Lett 1997; 225: 210–212.
Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP et al. Improved discrimination of Alzheimer's disease patients using beta-amyloid (1–42) and tau levels in CSF. Neurology 1999; 52: 1555–1562.
Green A, Harvey R, Thompson E, Rossor MN . Increased tau in the cerebrospinal fluid of patients with frontotemporal dementia and Alzheimer's disease. Neurosci Lett 1999; 259: 133–135.
McKhann G, Folstein M, Katzman R, Price D, Stadlan EM . Clinical diagnosis of Alzheimer's disease. Report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984; 34: 939–944.
Neary D, Snowden J, Gustafson L, Passant U, Stuss D, Black S et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998; 51: 1546–1554.
Will R, Alperovitch A, Poser S, Pocchiari M, Hofman A, Mitrova E et al. Descriptive epidemiology of Creutzfeldt–Jakob disease in six European countries, 1993–1995. EU collaborative study group for CJD. Ann Neurol 1998; 43: 763–767.
Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 1994; 44: 609–614.
Vanmechelen E, Vanderstichele H, Davidsson P, Van Kerschaver E, Van Der Perre B, Sjögren M et al. Quantification of tau phosphorylated at threonine 181 in human cerebrospinal fluid: a sandwich ELISA with a synthetic phosphopeptide for standardization. Neurosci Lett 2000; 285: 49–52.
Vandermeeren M, Mercken M, Vanmechelen E, Six J, Van de Voorde A, Martin JJ et al. Detection of tau proteins in normal and Alzheimer's disease cerebrospinal fluid with a sensitive sandwich enzyme-linked immunosorbent assay. J Neurochem 1993; 61: 1828–1834.
Herrmann M, Golombowski S, Kräuchi K, Frey P, Mourton-Gilles C, Hulette C et al. ELISA-quantitation of phosphorylated tau protein in the Alzheimer's disease brain. Eur Neurol 1999; 42: 205–210.
Hutton M, Lendon C, Rizzu P, Baker M, Froelich S, Houlden H et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393: 702–705.
Mann D, McDonagh A, Snowden J, Neary D, Pickering-Brown SM . Molecular classification of the dementias. Lancet 2000; 355: 626.
Sjögren M, Davidsson P, Tullberg M, Minthon L, Wallin A, Wikkelso C et al. Both total and phosphorylated tau are increased in Alzheimer's disease. J Neurol Neurosurg Psychiatry 2001; 70: 624–630.
Hesse C, Rosengren L, Andreasen N, Davidsson P, Vanderstichele H, Vanmechelen E et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett 2001; 297: 187–190.
Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, De Bernadis J et al. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer's disease patients. Neurosci Lett 2000; 287: 187–190.
Ishiguro K, Ohno H, Arai H, Yamaguchi H, Urakami K, Park JM et al. Phosphorylated tau in human cerebrospinal fluid is a diagnostic marker for Alzheimer's disease. Neurosci Lett 1999; 270: 91–94.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riemenschneider, M., Wagenpfeil, S., Vanderstichele, H. et al. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt–Jakob disease from other dementias. Mol Psychiatry 8, 343–347 (2003). https://doi.org/10.1038/sj.mp.4001220
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001220
Keywords
This article is cited by
-
Blood biomarkers for Alzheimer’s disease in clinical practice and trials
Nature Aging (2023)
-
P-tau subgroups in AD relate to distinct amyloid production and synaptic integrity profiles
Alzheimer's Research & Therapy (2022)
-
High altitude is associated with pTau deposition, neuroinflammation, and myelin loss
Scientific Reports (2022)
-
Neurodegenerative Disorders of Alzheimer, Parkinsonism, Amyotrophic Lateral Sclerosis and Multiple Sclerosis: An Early Diagnostic Approach for Precision Treatment
Metabolic Brain Disease (2022)
-
Comparison of cerebrospinal fluid tau, ptau(181), synuclein, and 14-3-3 for the detection of Creutzfeldt–Jakob disease in clinical practice
Journal of Neural Transmission (2022)