Abstract
The probability of suffering the mood disorder depression is up to 30% in women and 15% in men during their life span. Pharmacological options for depression are limited: conventional antidepressants have low efficacy and a delayed onset of action (several weeks). Here we investigate the antidepressant actions of inhibitors of monoacylglycerol lipase (MAGL), the major degradative enzyme of the endocannabinoid 2-arachidonoylglycerol. A low-dose of MAGL inhibitors produces antidepressant effects on acute stress-exposed mice, through glutamatergic synaptic long-term depression (LTD), without significant effects on chronic corticosterone-exposed mice. In contrast, a high-dose of MAGL inhibitors produces pro- or antidepressant effects on acute stress- or chronic corticosterone-exposed mice, respectively, through GABAergic synaptic disinhibition. In the hippocampus, in vivo inhibition of MAGL induces a CB1 cannabinoid receptor (CB1R)-dependent suppression of inhibitory GABAergic synapses and an in vivo LTD of excitatory glutamatergic synapses. LTD induction requires CB1R in astroglial cells (but not in GABAergic or glutamatergic neurons) and postsynaptic glutamate receptors. The conventional antidepressant fluoxetine produces rapid or delayed antidepressant effects in acute stress- or chronic corticosterone-exposed mice, respectively. We propose that depression-like behavior of animals in response to acute stress is the normal behavioral response, and thus, MAGL inhibitors, which produce antidepressant effects in chronic corticosterone-exposed animals through GABAergic synaptic disinhibition, represent a new class of rapidly-acting and long-lasting antidepressants.
Similar content being viewed by others
Introduction
Depression is a mood disorder characterized by low mood, feelings of despair, loss of pleasure and lack of motivation.1 As the probability of suffering depression is up to 30% in women and 15% in men during their life span,2 the conventional antidepressant serotonin/noradrenalin reuptake inhibitors have a delayed onset time of several weeks and low-remission rate.3 An acute dose of ketamine produces rapid antidepressant effects,4, 5 but over 1/3 of depressed patients do not respond to ketamine.5 It is therefore of great importance to identify new fast-acting antidepressants.
There are multiple lines of converging evidence supporting antidepressant potential of endogenous cannabinoids (endocannabinoids, eCBs):6, 7, 8 they are capable of reversing many effects of acute and chronic stress; they produce many biochemical signatures of antidepressants, such as neurogenesis and structural plasticity; impairments of eCB signaling in animals produce many behavioral effects akin to depression; humans with depression have lower levels of eCB; and large-scale clinical trials in Europe and the United States reported depressive effects in a significant subset of subjects following treatment with rimonabant, a selective antagonist of CB1 cannabinoid receptor (CB1R). There are two well characterized eCBs, anandamide or N-arachidonoylethanolamine and 2-arachidonoylglycerol (2-AG),9, 10, 11 with 2-AG 100- to 1000-fold more abundant than N-arachidonoylethanolamine in the brain.12 N-arachidonoylethanolamine and 2-AG are primarily hydrolyzed by fatty acid amide hydrolase and monoacylglycerol lipase (MAGL), respectively.9, 11 It is well established that eCBs can be rapidly synthesized postsynaptically and then released into the synaptic clefts to activate presynaptic CB1R, suppressing presynaptic release of the inhibitory and excitatory neurotransmitters GABA and glutamate, respectively.11 Thus, eCB activation of presynaptic CB1R can induce either short-term synaptic depression, that is, depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE), respectively, at GABAergic and glutamatergic synapses, or long-term depression (LTD), depending on its pattern of synthesis.11
Organisms react to stress by activating coordinated physiological and behavioral responses known as the stress response, which protects homeostasis and increases survival in the face of threatening environmental stimuli.13 These healthy allostatic responses are mainly regulated by the neuroendocrine hypothalamic–pituitary–adrenal axis.13 Over the past decades, ample evidence supports the notion that disruption of the hypothalamic–pituitary–adrenal axis leads to depression.14, 15 A single dose of the conventional antidepressant fluoxetine exerted rapid antidepressant behavioral effects in acutely stressed animals.16 In contrast, chronic but not sub-chronic administration of fluoxetine produced antidepressant effects in animals receiving chronic treatment with the stress hormone corticosterone (CORT) for 3 weeks.17 Because conventional antidepressants produce delayed antidepressant effects in humans,3, 17 these results suggest that the depression-like behavioral responses in acute stress- and chronic CORT-exposed animals represent normal stress responses and pathological depressive behaviors, respectively.
Recent studies suggest that increased N-arachidonoylethanolamine signaling has antidepressant effects in acutely stressed animals.7, 18 The MAGL inhibitor JZL184 (8 mg kg−1) did not produce significant antidepressant effects in chronically stressed animals,19 although its daily injection before daily chronic stress prevented the establishment of behavioral depression in an animal model.19, 20 Motivated by our pilot experiments, here we investigated the hypothesis that different doses of the MAGL inhibitors JZL184 and KML29 produce pro- or antidepressant effects on acutely stressed and chronic CORT-treated mice through distinct mechanisms.
Materials and Methods
Animals
All procedures were performed in keeping with the guidelines established by the Canadian Council on Animal Care as approved by Animal Care Committee of the University of Ottawa Institute of Mental Health Research. Specifically, the IMHR ACC approved the present study (ACC-2012-004). Similar procedures were approved by the Fourth Military Medical University, China.
Behavioral study (investigators blinded to the group allocation) employed male CD1 (30-35 g) or 8-w C57BL/6 mice (Charles Rivers, Montreal, QC, Canada). In vitro or in vivo electrophysiological study employed male Sprague–Dawley rats (Charles River) weighing 75–100 or 220–250 g, respectively. Mice and rats were housed in groups of 4 and 2, respectively, and maintained under 12h/12 h light/dark cycle, 22±2 °C, food and water ad libitum.
Mutant mice were generated by Beijing Biocytogen, China. Briefly, from BAC library, the 7.4-kb of genomic fragment upstream of the CNR1 exon 2 and the 9.5-kb frangment downstream were used as 5′ and 3′ homologous regions, respectively. The targeting vector included the 5′ region, a loxP site (the CNR1 exon 2, a neomycin phosphotransferase-expression cassette with two Frt sites flanked), a second loxP site and the 3′ region. After digested by AscI, the linearized vector was electroporated to embryonic stem cells. Positive clones were screened by G418 and then identified by Southern blot. Selected embryonic stem clones were injected into blastocysts of C57BL/6 mice. Chimera mice were crossed with wild-type mice. Floxed and wild-type CNR1 alleles were detected by PCR using four primers: CNR1-A1 LoxP-F: 5′-ACTGGACAGCTCATCCCTTG-3′; CNR1-A1 LoxP-R: 5′-AAGTCAATGGTCTTGCATGGATCT-3′; CNR1-Frt-F: 5′-AGGCAACACTCAGT GACAGC-3′; CNR1-Frt-R: 5′-TAGGTGGATCTCAGGAATCTTGTAG-3′. CNR1-A1 LoxP-F and CNR1-A1 LoxP-R were used to detect the first loxP site, and the wild-type and floxed alleles were 244 and 301 bp, respectively. CNR1-Frt-F and CNR1-Frt-R were for a second loxP site, with the wild-type and floxed alleles of 254 and 369 bp, respectively. As described in our recent study,21 CB1R-floxed mice were crossed with three mouse lines: transgenic mice expressing the inducible version of the Cre recombinase CreERT2 under the control of the human glial fibrillary acidic protein promoter (GFAP-CreERT2 mice)22 to obtain a GFAP-CB1R-KO mouse line; the Dlx5/6-Cre transgenic mice having Cre recombinase expression directed by the regulatory sequences of the zebrafish dlx5a/dlx6a genes_ENREF 2 (ref. 23) to obtain the GABA-CB1R-KO mouse line; or the CaMKIIa-iCre transgenic mice expressing the improved Cre in glutamatergic pyramidal cells of the hippocampus24, 25, 26 to obtain the Glu-CB1R-KO mouse line. Deletion of CB1R gene from GFAP-CB1R-KO mouse was obtained in mice (8 weeks) by eight daily injections of tamoxifen (Sigma, St. Louis, MO, USA; 1 mg, intraperitoneal (i.p.)).21 CB1R protein deletion in mutant mice was verified by immunohistochemistry for electron microscopy.
CB1R immunohistochemistry for electron microscopy
Mice were transcardially perfused under anesthesia with 100 ml of 0.1% glutaraldehyde, 4% formaldehyde and 15% picric acid. Coronal hippocampal vibrosections were cut at 50 μm. After freeze-thawed with liquid nitrogen, the sections were incubated for 1 h in 0.05 m Tris-HCl-buffered saline (pH 7.4), for 24 h in primary polyclonal rabbit anti-CB1R (1:100) antibody with or without monoclonal mouse anti-GFAP antibody (1:1000; Chemicon, Temecula, CA, USA), and overnight in biotinylated donkey anti-rabbit IgG (1:200, Millipore, CA, USA) with or without 1.4-nm gold-particle-conjugated goat ant-rabbit IgG (1:100, Nanoprobes, Stony Brook, NY, USA). For double labeling, sections were postfixed with 1% glutaraldehyde and enhanced with an HQ Silver Kit (Nanoprobes). All the sections were incubated in avidin–biotin-peroxidase complex (1:50, Vector Lab, Burlingame, CA, USA) for 6 h, in 0.02% 3,3′-diaminobenzidine-4HCl and 0.003% (v/v) H2O2 for 20–30 min, in 1% OsO4 for 1 h and finally in 1% uranyl acetate for 1 h. After dehydration, the sections were mounted onto glass slides and embedded in epoxy resin. Samples were cut into 60-nm-thick sections, which were mounted on grids and examined with a JEM-1400 electron microscope (JEM, Tokyo, Japan). Positive labeling was visualized and counted. GFAP-stained cells were considered astrocytes, whereas presumed GABAergic or glutamatergic nature of an axon was established by the presence of symmetric or asymmetric synapses, respectively.
Behavioral tests
Mouse surgery
Under isoflurance anesthesia, mouse was implanted with two guide cannulae (21 Ga) into the bilateral CA1 area (B/P −2.0, M/L ±1.6, D/V −1.5 mm). One week later, intra-CA1 microinjection (0.5 μl/side) was performed. After behavioral test, mice were anesthetized and perfused transcardially with 4% paraformaldehyde, and brains were cut for cresyl violet staining to confirm correct cannula placement.
Forced-swimming test
Mice were placed into a Plexiglas cylinder (65 × 30 cm, filled with 25 °C swater to 10-cm height) for 5 min, during which the duration of immobility, defined as remaining motionless, was quantified with an automated behavioral tracking system (View Point Life Sciences, Montreal, QC, Canada).27
Novelty-suppressed feeding test
After food deprivation for 12 h, mouse was placed in a corner of the novel box (45 × 45 × 45 cm) with chow in the center of the box.27 The latency to begin eating or chewing the food was measured. If mice had not eaten within 480 s, their latency was taken to be 480 s. Then, mice were transferred to their home cages for the measurement of the latency to feed with a cutoff of 8 min.
Sucrose preference test
After 12-h water deprivation, mouse was allowed 1 h access to one bottle of water and one bottle of 1% sucrose in its home cage. Sucrose consumption volume was divided by the total volume of water and sucrose consumption.
Open field test
Locomotor activity was measured, in a dimly lighted room, for 5 min in an apparatus (40 × 40 × 40 cm) equipped with photobeam arrays (12 × 12). Mouse movement interrupted infrared photobeams, which was quantified by a computer.
Elevated-plus maze
Immediately after open field test, elevated-plus maze was conducted, also in the dimly lighted room, in an apparatus consisting of a central area (7 × 7 cm) connecting to two open arms (40 × 7 cm) and two closed arms (40 × 7 cm) with side and end walls of 10 cm in height. A mouse was placed on the center area with its head facing an open arm for 5 min. Anxiety behavior was measured by analyzing the percentage of time in open arm and percentage of open arm entries.
Motor balance test
Immediately after elevated-plus maze test, the mouse was placed on a 100 cm × 12 mm beam resting 50 cm above the tabletop. A black box was placed at the end of the beam as the finish point. A lamp above the starting point served as an aversive stimulus. The time for the mouse to traverse the beam was measured.
Drug treatment protocol
Drugs were purchased from Tocris Bioscience, Ellisville and dissolved in 10% Tween-80, 10% dimethyl sulphoxide and 80% physiological saline, if otherwise stated. To study the effects of acute stress, mice received: (I) AM281 (0.3, 1 or 3 mg kg−1, i.p.) or vehicle 30 min before forced swim test (FST); (II) JZL184 (2.5, 5, 10 or 20 mg kg−1, i.p.), Tat-GluR2 (GL Biochem, Shanghai, China; 1.5 μmol kg−1, i.p., in physiological saline), or vehicle 2 h before FST or novelty-suppressed feeding; (III) KML29 (5, 10, 20 or 40 mg kg−1, i.p.) or vehicle 4 h before FST, (IV) AM281 (0.3 mg kg−1, i.p.), muscimol (Sigma; 0.05 mg kg−1, i.p., in physiological saline) or vehicle 10 min before JZL184, fluoxetine or ketamine (Sigma; 10 mg kg−1, i.p., in physiological saline), followed 30 min (ketamine) or 2 h (JZL184) later by FST; (V) Tat-GluR2 or Tat-GluR2s (1.5 μmol kg−1, i.p.; 15 pmol/per side of the CA1) 2 h (i.p.) or 1 h (intra-CA1) before JZL184 or 10 min before KML29, followed 2 h (JZL184) or 4 h (KML29) later by FST; (VI) Tat-GluR2 or Tat-GluR2s (1.5 μmol kg−1, i.p.) 2 h before and muscimol (0.05 mg kg−1, i.p.) or 30 min before JZL184 (20 mg kg−1, i.p.), followed 2 h later by FST; or (VII) Tat-GluR2 or Tat-GluR2s (1.5 μmol kg−1, i.p.) 10 min before KML29, followed 3.5 h and 4 h later by muscimol (0.05 mg kg−1, i.p.) and FST, respectively. Open field test, elevated-plus maze, motor balance test and FST were performed in sequence after JZL184, intra-CA1 infusion of Tat-GluR2 or Tat-GluR2s 1 h before JZL184, or muscimol (0.05 mg kg−1, i.p.) 10 min before JZL184 (20 mg kg−1, i.p.). Mutant mice received JZL184 (20 mg kg−1, i.p.) or vehicle 2 h before FST. For sucrose preference test (SPT), mice were deprived of water for 12 h before receiving JZL184 (5 mg kg−1, i.p.) or vehicle, as other mice received Tat-GluR2 or Tat-GluR2s (1.5 μmol kg−1, i.p.) 1 h before JZL184, followed 2 h later by SPT.
To study the effects of chronic CORT exposure, mice received CORT solution or vehicle in place of normal drinking water for 21 days.17, 28 Then the mice sequentially received water for 2 days, 1% sucrose for 2 days, one bottle of water and one bottle of 1% sucrose for 1.5 days, and water deprivation for 12 h, followed by weighing the mice before FST or SPT. To study antidepressant effects, mice received JZL184 (5 or 20 mg kg−1, i.p.), KML29 (10 or 40 mg kg−1, i.p.) or vehicle with or without muscimol (0.05 mg kg−1, i.p.) or vehicle pretreatment, followed by 12 h later by water deprivation for SPT, 24 h before FST or 8 days before SPT. To study the effects of fluoxetine, 2 days after CORT treatment mice received fluoxetine (10 mg kg−1, i.p.) or vehicle once or once per day for 5 or 21 days, followed 1 day later by FST. To study the effects of repeated JZL184 injection, 1 day after CORT treatment mice received 3 JZL184 (20 mg kg−1, i.p.) or vehicle injections once every 5 days, followed 1 day later by FST. To study the effects of JZL184 and ketamine on chronic CORT-treated mice in response to acute stress, mice received JZL184 (20 mg kg−1, i.p.), ketamine (10 mg kg−1, i.p.) or vehicle at 12 h before water deprivation, followed sequentially by FST and SPT.
Electrophysiology analysis
Patch clamp of hippocampal slices
Rats received JZL184 (5 or 20 mg kg−1, i.p.) or vehicle with or without AM281 (3 mg kg−1, i.p.) or vehicle, followed 45 min later by decapitation for preparation of hippocampal slices (400 μm) that were placed in ice-cold artificial cerebral spinal fluid. During recording, the slices were perfused with artificial cerebral spinal fluid at 30 °C. To record postsynaptic currents (PSCs), neurons were whole-cell voltage clamped at −70 mV. To record miniature inhibitory postsynaptic currents (mIPSCs), the patch pipette was filled with a solution (in mm: 120 KCl, 20 K gluconate, 10 HEPES, 10 phosphocreatine Na salt, 2 adenosine triphosphate (ATP) Na salt, 0.4 guanosine-5'-triphosphate (GTP) Na salt, and 2 MgCl2, pH 7.35), as ASCF was supplemented with 1 μm tetrodotoxin (TTX), 10 μm 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50 μm D-(-)-amino-5-phosphonopentanoic acid (D-APV). To record miniature excitatory postsynaptic currents (mEPSCs), the patch pipette was filled with a solution (in mm: 120 K gluconate, 20 KCl, 10 HEPES, 10 phosphocreatine Na salt, 2 ATP Na salt, 0.4 GTP Na salt, and 2 MgCl2, pH 7.35), as ASCF was supplemented with 1 μm TTX and 10 μm bicuculline. To induce DSI or DSE, cells were depolarized from −70 to 0 mV for 5 s, and IPSCs/EPSCs were evoked at 4-s intervals. To study paired pulse facilitation (PPF), a stable whole-cell recording configuration was established on a CA1 pyramidal cell and a second stimulation glass electrode (2–6 MQ) filled with 2M NaCl was lowed into the stratum radiatum. PSCs were collected by voltage clamp recordings using a stimulus pulse with a single square wave. Paired-pulse responses were performed at intervals 100 ms. PPF ratio was measured as the amplitude ratio of the second to the first postsynaptic response from an average of 20–30 pairs of pulses. The data were acquired with pCLAMP 10.4 (Molecular Devices, Sunnyvale, CA, USA), measured and plotted with pCLAMP 10.0 and GraphPad prism 6.0. Mini-analysis software (Synaptosoft, Fort Lee, NJ, USA) was used for analyses of PSCs and generation of cumulative probability plots.
Electrophysiology in anesthetized animals
The detailed method was described in our recent study.21 Briefly, electrodes were inserted into the CA1 area with mouse coordinates (B/P −2, M/L −1.5, D/V 1.3 mm for recording electrode, and B/P +1.9, M/L +2.2, D/V −1.3 mm for stimulating electrode with insertion angle of 20°) and rat coordinates (B/P −4, M/L −2.8, D/V 2.5 mm for recording electrode, and B/P +3.5, M/L +3.2, D/V −2.4 mm for stimulating electrode with insertion angle of 20°). After recording of baseline field excitatory postsynaptic potentials (fEPSPs) for 20 min, animals received different treatment before fEPSP recording for 120 min: (I) JZL184 (5 or 20 mg kg−1, i.p.), fluoxetine (10mg kg−1, i.p.) or vehicle; (II) Ro25,6981 (Sigma; 6 mg kg−1, i.p.; dissolved in physiological saline) or vehicle 10 min before JZL184; (III) Tat-GluR2 or Tat-GluR2s (1.5 μmol kg−1, i.p.) 2 h before JZL184; (IV) intra-CA1 iontophoretic ejection (−40 nA for 30 sec) of 2-AG (50 μg ml−1) or vehicle; (V) actinomycin-D (Sigma; 72 μg/12 μl, intracerebroventricular injection; dissolved in 10% Tween-80, 20% dimethyl sulfoxide and 70% physiological saline) or vehicle 2 h before a 2-AG ejection.
Statistical analysis
The sample size of this study was determined according to our previous studies.21, 27 Data were reported as mean±s.e.m. Statistical analysis of the data was performed using the Student’s t test, χ2 test, one-way analysis of variance (ANOVA) or two-way ANOVA for repeated-measures, followed by the least significant difference (LSD) post-hoc test. Statistical significance was set at P<0.05.
Results
Biphasic effects of MAGL inhibitors on acutely stressed mice
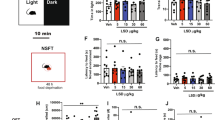
Injection of 5 or 20 mg kg−1 JZL184 significantly decreased or increased immobility in the FST, respectively, in naïve mice (Figure 1a), without significant effects on basal locomotor activity, anxiety level or motor balance tested before the FST (Supplementary Figures 1a–d). Pretreatment with the CB1R antagonist AM281 at a dose (0.3 mg kg−1, i.p.) that did not significantly affect immobility (Figure 1b) blocked the behavioral responses of both 5 and 20 mg kg−1 JZL184 (Figures 1c and d). Both fluoxetine (10 mg kg−1, i.p.) and ketamine (10 mg kg−1, i.p.) also significantly decreased immobility, which, however, was not affected by AM281 pretreatment (Supplementary Figures 2a and b). As traditional antidepressants were effective in the NSF model of anxiety, 5 or 20 mg kg−1 JZL184 respectively decreased or increased the latency to eat food in a novel environment (Supplementary Figures 2c and d). These results suggest that 2-AG action on CB1R of acutely stressed mice mediates the biphasic effects of low- and high-doses JZL184, but not the immobility-decreasing effects of fluoxetine and ketamine.
Biphasic effects of JZL184 on immobility of acutely stressed mice (a) an intraperitoneal (i.p.) injection of 5 or 20 mg kg−1 of JZL184 respectively decreases and increases immobility. (b) AM281 dose-dependently decreases immobility. (c–f) An i.p. injection of AM281 (0.3 mg kg−1) (c,d) or Tat-GluR2 (e) and an intra-CA1 injection of Tat-GluR2 (f) abolish the decreased immobility produced by JZL184 (5 mg kg−1, i.p.). (g) Muscimol and JZL184 (20 mg kg−1) together decrease immobility. (h) Tat-GluR2 and muscimol together abolish the increased immobility produced by 20 mg kg−1 JZL184. All summary graphs show means±s.e.m.; n=numbers of animals recorded in each group. *P<0.05 and **P<0.01 vs control, LSD post-hoc test after one-way ANOVA (a: F4,51=10.173, P<0.01; b: F3,42=12.573, P<0.01; c: F3,40=12.098, P<0.01; d: F3,40=9.817, P<0.01; e: F4,51=9.212, P<0.01; f: F2,32=13.189, P<0.01; g: F3,40=20.173, P<0.01; h: F3,42=9.107, P<0.01).
Because synthetic cannabinoids induced in vivo LTD at CA3-CA1 synapses,21 we examined the possible involvement of such LTD in JZL184 effects on immobility. An i.p. injection of the LTD-blocking peptide Tat-GluR2 abolished the decreased immobility effects of JZL184 (5 mg kg−1, i.p.) as Tat-GluR2 alone did not significantly affect immobility (Figure 1e). An intra-CA1 infusion of Tat-GluR2 also abolished JZL184-decreased immobility (Figure 1f) without significant affects on basal locomotor activity, anxiety level or motor balance (Supplementary Figures 1e–h). Similarly, JZL184 (5 mg kg−1, i.p.) prevented acute stress-induced decrease of sucrose consumption (Figure 2a), which was abolished by Tat-GluR2 (1.5 μmol kg−1, i.p.) (Figure 2b).
Biphasic effects of monoacylglycerol lipase (MAGL) inhibitors on acutely stressed mice (a, b) JZL184 (5 mg kg−1, intraperitoneal (i.p.)) in naïve mice prevents acute stress-decreased sucrose consumption (a), which is abolished by Tat-GluR2 (b). (c) An i.p. injection of 10 or 40 mg kg−1 KML29 decreases and increases immobility, respectively. (d) Tat-GluR2 abolishes decreased immobility by 10 mg kg−1 KML29. (e) Tat-GluR2 and muscimol together abolishes increased immobility by 40 mg kg−1 KML29. (f) Vehicle or JZL184 (20 mg kg−1, i.p.) induces comparable immobility in GFAP-CB1R-WT and GFAP-CB1R-KO mice, with an increase of immobility produced by JZL184. (g) JZL184 (20 mg kg−1, i.p.) increases immobility in GABA-CB1R-WT mice. (h) Vehicle or JZL184 (20 mg kg−1, i.p.) induces comparable immobility in Glu-CB1R-WT and Glu-CB1R-KO mice, with an increase of immobility produced by JZL184. All summary graphs show means±s.e.m.; n=numbers of animals recorded in each group. *P<0.05 and **P<0.01 vs control, LSD post-hoc test after one-way ANOVA (a: F3,38=4.651, P<0.01; b: F2,33=0.521, P<0.05; c: F4,48=10.183, P<0.01; d: F2,31=15.772, P<0.01; e: F3,39=3.361, P<0.05) or two-way ANOVA (f: F1,32=10.511, P<0.01; treatment F=20.873, P<0.01; g: F1,32=9.883, P<0.01; genotype F=19.731 P<0.01; h: F1,32=6.725, P<0.01; treatment F=12.314, P<0.01).
If 5 mg kg−1 JZL184-decreased immobility through LTD induction, an opposite mechanism may underlie increased immobility of 20 mg kg−1 JZL184. We reasoned that 20 mg kg−1 JZL184 might preferentially activate GABAergic presynaptic CB1R to disinhibit (i.e., excite) postsynaptic neurons, leading to increased immobility. Consistent with our hypothesis, the GABA-A receptor agonist muscimol (0.05 mg kg−1, i.p.), which did not significantly affect immobility (Supplementary Figure 2e), before 20 mg kg−1 JZL184-decreased immobility (Figure 1g) without significant effects on basal locomotor activity, anxiety level or motor balance examined before FST (Supplementary Figures 1i–l). Tat-GluR2 did not significantly affect immobility of naïve mice treated with 20 mg kg−1 JZL184 (Supplementary Figure 2f), but pretreatment with both muscimol (0.05 mg kg−1, i.p.) and Tat-GluR2 (1.5 μmol kg−1, i.p.) before JZL184 completely blocked the increased immobility effects of 20 mg kg−1 JZL184 (Figure 1h).
We then studied KML29, a more selective MAGL inhibitor than JZL184.9 Treatment of naïve mice with 10 or 40 mg kg−1 KML29 at 4 h9 before FST significantly decreased and increased immobility, respectively (Figure 2c). Tat-GluR2 pretreatment abolished the decreased immobility effects of 10 mg kg−1 KML29 (Figure 2d), and pretreatment with both Tat-GluR2 and muscimol abolished the increased immobility effects of 40 mg kg−1 KML29 (Figure 2e).
Next, we investigated mutant mice by generating CB1R-floxed mice (Supplementary Figure 3), GFAP-CB1R-KO mouse line (Supplementary Figure 4), GABA-CB1R-KO mouse line (Supplementary Figure 5) and Glu-CB1R-KO mouse line (Supplementary Figure 6). Relative to vehicle that induced comparable immobility between GFAP-CB1R-WT and GFAP-CB1R-KO mice (Figure 2f), 20 mg kg−1 JZL184 significantly increased immobility in both GFAP-CB1R-WT and GFAP-CB1R-KO mice, without significant differences between them (Figure 2f). Both GABA-CB1R-WT and GABA-CB1R-KO mice showed comparable immobility in response to vehicle (Figure 2g), which, in agreement with recent studies,29, 30 suggests that mouse behavioral coping to acute stress does not require CB1R in GABAergic neurons. Relative to vehicle injection, however, JZL184 (20 mg kg−1, i.p.) significantly increased immobility in GABA-CB1R-WT but not GABA-CB1R-KO mice (Figure 2g), suggesting the requirement of CB1R in GABAergic neurons for 20 mg kg−1 JZL184 to increase immobility. Both Glu-CB1R-WT and Glu-CB1R-KO mice showed comparable immobility in response to vehicle or JZL184, with a significant increase of immobility after JZL184 (Figure 2h), which is in agreement with a recent study,29 suggesting that behavioral coping to acute stress does not require CB1R or 2-AG signaling in glutamatergic synapses.
Opposite effects of MAGL inhibitors on chronic CORT-exposed mice
After drinking CORT water for 21 days, mice showed significantly increased immobility (Figure 3a) and decreased sucrose intake (Figure 3d) without a significant change in body weight (Supplementary Figure 2g). Injection of 20 but not 5 mg kg−1 JZL184 significantly decreased immobility (Figure 3b) and increased sucrose intake (Figure 3e) 1 day after JZL184 injection. As the decreased immobility was abolished by muscimol pretreatment (Figure 3c), the increased sucrose intake lasted for at least 1 week (Figure 3f), and 3 JZL184 injections (20 mg kg−1) once every 5 days still significantly decreased immobility (Figure 3g). A single injection of 40 but not 10 mg kg−1 KML29 also significantly decreased immobility (Figure 3h).
Antidepressant effects of MAGL inhibitors, fluoxetine and ketamine on chronic CORT-treated mice (a, d) Chronic CORT-treated mice show increased immobility (a) and decreased sucrose consumption (d). (b, c, e, f) An intraperitoneal (i.p.) injection of 20 but not 5 mg kg−1 JZL184 decreases immobility (b) and increases sucrose intake (e), as the decreased immobility is blocked by muscimol pretreatment (c) and lasts for 1 week (f). (g) JZL184 (20 mg kg−1, i.p., 3 × 1/5 days) decreases immobility. (h) An i.p. injection of 40 but not 10 mg kg−1 KML29 decreases immobility. (i) Chronic but not a single or sub-chronic injection of fluoxetine (10 mg kg−1, i.p.) decreases immobility. (j, k) An i.p. injection of 10 mg kg−1 ketamine decreases immobility (j) without significant effects on sucrose intake (k) in mice in response to acute stress, whereas JZL184 (20 mg kg−1, i.p.) increases sucrose intake in mice in response to acute stress (k). All summary graphs show means±s.e.m.s; n=numbers of animals recorded in each group. *P<0.05 and **P<0.01 vs control, #P<0.01 vs vehicle, t test (a, d) or LSD post-hoc test after one-way ANOVA (b: F3,36=27.001, P<0.01; c: F2,27=7.426, P<0.01; e: F3,42=24.974, P<0.001; f: F2,33=26.956, P<0.01; g: F2,27=12.301, P<0.05; h: F3,36=21.890, P<0.01; i: F3,36=39.231, P<0.05; j: F2,27=12.505, P<0.001; k: F4,70=41.114, P<0.01).
In contrast, once daily chronic but not a single or once daily sub-chronic injection of fluoxetine (10 mg kg−1, i.p.) significantly decreased immobility (Figure 3i). An acute injection of ketamine (10 mg kg−1, i.p.) significantly decreased immobility (Figure 3j) without significant effects on sucrose intake of chronic CORT-treated mice in response to acute inescapable stress (Figure 3k). However, 20 mg kg−1 JZL184 significantly increased sucrose intake in chronic CORT-treated mice in response to acute stress (Figure 3k).
JZL184 enhances 2-AG signaling at CA1 GABAergic synapses
Our behavioral data suggest JZL184-increased 2-AG signaling at CA1 GABAergic synapses. To provide compelling evidence in support of these findings, we recorded mIPSCs and IPSCs of CA1 pyramidal neurons in hippocampal slices prepared 45 min after JZL184 injection. An i.p. injection of 20 mg kg−1 JZL184 not only significantly decreased mIPSC frequency in a CB1R-dependent manner without significant effects on mIPSC amplitude (Figures 4a–c; Supplementary Figures 7a and b), but also significantly increased the PPF ratio of IPSCs in a CB1R-dependent manner (Figures 4d and e). Relative to vehicle-treated mice, mice treated with 20 mg kg−1, but not 5 mg kg−1 JZL184 showed a significantly decreased DSI magnitude (Figure 4f; Supplementary Figures 7e and f). However, both mEPSCs and DSE remained unchanged after 20 mg kg−1 JZL184 treatment (Figures 4g–i; Supplementary Figures 7c and d). These data suggest that 20 but not 5 mg kg−1 JZL184 enhance 2-AG signaling at CA1 GABAergic but not glutamatergic synapses in living animals.
JZL184 reduces CA1 presynaptic GABA release (a–e) Histogram (left) and representative traces (right) show that relative to control (Con), a JZL184 injection (J 20: 20 mg kg−1, intraperitoneal (i.p.)), but not vehicle (Veh), decreases miniature inhibitory postsynaptic current (mIPSC) frequency (a) and increases paired pulse facilitation (PPF) ratio of inhibitory postsynaptic current (IPSC) (d), which are abolished by AM281 pretreatment (b, e), without significant effects on mIPSC amplitude (c). (f) JZL184 significantly decreases the magnitude (P<0.05) of depolarization-induced suppression of inhibition (DSI) that is induced by 5-s depolarization from −70 to 0 mV. Representative IPSC traces are superimposed on the top. (g, h) Histograms show that JZL184 does not significantly affect miniature excitatory postsynaptic current (mEPSC) frequency (g) or amplitude (h). (i) JZL184 does not significantly affect the magnitude (P=0.17) of depolarization-induced suppression of excitation (DSE), which is induced by 5-s depolarization from −70 to 0 mV. Representative excitatory postsynaptic current (EPSC) traces are superimposed on the top. *P<0.05 and **P<0.01 vs control, LSD post-hoc test after one-way ANOVA (a: F2,13=6.181, P<0.05; b: F2,12=0.278, P=0.762; c: F2,13=0.207, P=0.816; d: F2,14=10.91, P<0.01; e: F2,13=0.034, P=0.967) or t test (f–i).
JZL184 induces in vivo LTD at CA3-CA1 synapses
Our behavioral data also suggest JZL184-induced in vivo LTD at glutamatergic CA3-CA1 synapses. This idea is supported by our findings that both 5 and 20 mg kg−1 JZL184, but not fluoxetine (10 mg kg−1, i.p.), significantly decreased fEPSP slope (Figures 5a and h; Supplementary Figure 8). Intra-CA1 iontophoretic application of 2-AG induced a similar synaptic depression (Figures 5b and h), and the RNA transcription inhibitor actinomycin-D31 blocked the late but not the early phase of LTD expression (Figures 5c and h).
JZL184 induces in vivo long-term depression (LTD) at CA3-CA1 synapses. (a–g) Plots of normalized field excitatory postsynaptic potential (fEPSP) slopes in anesthetized mice (a, d–g) or rats (b, c) show that an administration of JZL184 (a, d–g) or 2-AG (b, c) at 0 min elicits LTD lasting for >2 h in wild-type (a, d–g), Glu-CB1R-KO and GABA-CB1R-KO mice (e), but not in GFAP-CB1R-KO mice (d), which is blocked by Ro25,6981 (f) and Tat-GluR2 (g), as actinomycin-D blocks the late-phase expression of 2-AG-elicited LTD (c). Representative fEPSP traces before (1) and after (2) vehicle, JZL184, or 2-AG administration are shown below each plot. (h) Histogram summarizes the average percent change of fEPSP slope before (1) and after (2) vehicle, JZL184 or 2-AG administration as depicted in a–g. All summary graphs show means±s.e.m.s; n=numbers of animals recorded in each group (a–g). *P<0.05 and **P<0.01 vs control, t test (b–d, f, g) or LSD post-hoc test after one-way ANOVA (a: F2,9=31.904, P<0.01; e: F2,6=27.102, P=0.892).
Our recent study showed that synthetic cannabinoid-induced in vivo LTD at CA3-CA1 synapses required activation of astroglial CB1R, activation of postsynaptic NR2B-containing N-methyl-d-aspartic acid receptor (NR2BR) and endocytosis of postsynaptic α-amino-3-hydroxy-5-methyl-isoxazole propionic acid receptors (AMPAR).21 Here we observed similar results: JZL184 (20 mg kg−1, i.p.) induced LTD at CA3-CA1 synapses from GFAP-CB1R-WT mice but not from GFAP-CB1R-KO littermates (Figures 5d and h) and JZL184-elicited CA1 LTD was indistinguishable between wild-type mice and GABA-CB1R-KO or Glu-CB1R-KO littermates (Figures 5e and h); the NR2BR antagonist Ro25-6981 (6 mg kg−1, i.p.)21 and the AMPAR endocytosis-blocking peptide Tat-GluR2 (1.5 μmol kg−1, i.p.)21 blocked JZL184-induced in vivo LTD at CA3-CA1 synapses (Figures 5f–h).
Discussion
Stress plays a key role in the etiology and/or exacerbation of major depression.32 The patterns of animal behavioral coping in response to acute inescapable stress in FST has been widely and successfully used to examine antidepressant efficacy, where less or more immobility indicates active or passive behavioral coping to acute stress challenge, respectively. We therefore performed FST in naïve animals to examine whether 2-AG accumulation by JZL184-decreased or increased immobility, that is, anti or pro-depressant behavioral responses of mice in response to acute stress. We found that 5 or 20 mg kg−1 JZL184 and 10 or 40 mg kg−1 KML29 respectively produced anti- or pro-depressant responses in a CB1R dependent manner. We further showed that the antidepressant effects of both JZL184 and KML29 can be blocked by either systemic or intra-CA1 injection of the AMPAR endocytosis-blocking peptide Tat-GluR2, suggesting the requirement of astroglial-mediated LTD at CA3-CA1 synapses for low-dose MAGL inhibitors to produce antidepressant effects in acutely stressed mice. We studied the hippocampal CA1 area because JZL184-elicited antidepressant effects were found to be associated with CA1 area.19
Loss of pleasure (i.e., anhedonia) is one of the core symptoms of patients with major depression.1 We observed that JZL184 (5 mg kg−1, i.p.) prevented acute stress-induced decrease of sucrose consumption, that is, anti-anhedonia behavioral response, which was abolished by Tat-GluR2. Therefore, LTD expression is also necessary for 5 mg kg−1 JZL184 to produce anti-anhedonia effects in acutely stress mice. Employing both pharmacological strategy and mutant mice, we found results suggesting that high dose JZL184 and KML29 both induce LTD at CA3-CA1 synapses and activate CA1 GABAergic presynaptic CB1R, but the latter action overrides the former, so as to produce pro-depressant behavioral response.
The anti and pro-depressant effects produced respectively by low- and high-doses of MAGL inhibitors on acutely stressed mice are unlikely produced by JZL184 action on motor activity and anxiety. This is because neither dose-significantly-affected locomotor activity and anxiety measures tested before the FST. Recent studies reported anxiolytic effects of 8 or 16 mg kg−1 of JZL184,33, 34, 35 which was likely produced under high, but not low, levels of environmental aversiveness.35 We performed both the FST and other behavioral tests in a low level of environmental aversiveness.
Environmental stress manipulations in rodents, such as chronic unexpected-mild stress (CUMS) and repeated social defeat, produce protocol variability and frequently reported difficulties in replication.17 In contrast, chronic oral exposure to the stress hormone CORT induced persistent and long-lasting depression-like behaviors in rodents that could be reversed with chronic, but not sub-chronic, administration of conventional antidepressants.17 We confirmed here that chronic CORT-treated mice showed significant pro-depressant and anhedonia behavioral responses, which was abolished by chronic but not a single or sub-chronic injection of the conventional antidepressant fluoxetine. JZL184 (8 mg kg−1) did not produce significant antidepressant effects in mice receiving CUMS for 5 weeks,19 although its daily injection before daily CUMS prevented the establishment of animal model of behavioral depression.19, 20 These results predict a dismal future for the use of JZL184 to treat depression, because it is not practical to administer an antidepressive therapy before the onset of depression. However, we showed here that 20 mg kg−1 JZL184 and 40 mg kg−1 KML29 produced significant antidepressant behavioral responses in chronic CORT-treated mice, thus opening a window for the clinical treatment of depression with MAGL inhibitors. Interestingly, acute administration of 20 mg kg−1 JZL184 to chronic CORT-exposed mice produced rapid and long-lasting antidepressant effects, that is, onset with 1 day and lasting for at least 7 days. Although once daily JZL184 injection (40 mg kg−1 i.p.) for 6 days mimics a partial CB1R antagonism attributable to CB1R downregulation,36 and sub-chronic JZL184 injection (8 mg kg−1, i.p.) did not produce significant antidepressant effects in CUMS mice,19, 37 we found here that three JZL184 injections (20 mg kg−1) once every 5 days still produced significant antidepressant effects.
The present finding that 5 mg kg−1 JZL184-induced antidepressant effects in acutely stressed mice without significant effects on chronic CORT-treated mice suggests opposite mechanisms underlying depression-like behaviors of acute and chronic stress-exposed mice. In support of this idea, 20 mg kg−1 JZL184 produced pro- and antidepressant behavioral responses, respectively, in acute stress and chronic CORT-exposed mice, both of which are likely achieved via disinhibition of GABAergic synapses. Indeed, acute stress38 and chronic stress-exposed rats,39 respectively, showed enhanced and suppressed DSI of CA1 pyramidal neurons. These lines of evidence suggest that depression-like behaviors of acute and chronic stress-exposed mice result from enhancement and suppression of CA1 pyramidal neurons, respectively. Thus, our results can be plausibly explained: a low-dose of MAGL inhibitors produces antidepressant effects on acute stress-exposed mice without significant effects on chronic CORT-treated mice, because this low-dose elicits LTD at glutamatergic synapses, hence suppressing CA1 pyramidal neurons; in contrast, a high-dose of MAGL inhibitors produces pro- and antidepressant effects on acute stress and chronic CORT-exposed mice, respectively, because this high-dose disinhibits GABAergic synapses, hence exciting CA1 pyramidal neurons.
Clinical studies have shown fast antidepressant effects of subanesthetic dose of ketamine on treatment-resistant depressed patients.4, 5 However, ketamine can produce neurotoxicity in rodents and psychotomimetic effects in humans,4 and 1/3 depressed patients did not respond to ketamine.5 As ketamine produced rapid antidepressant effects in both acutely and chronically stressed animals,40, 41 we found that JZL184 produced pro- and antidepressant effects in acute stress and chronic CORT-exposed mice, respectively, suggesting different mechanisms underlying the fast antidepressant effects of ketamine and MAGL inhibitors. This idea is supported by our data that AM281 blocked JZL184 but not ketamine effects. Interestingly, JZL184 but not ketamine induced anti-anhedonia effects in chronic CORT-treated mice in response to acute stress.
Recent studies showed that application of JZL184 to hippocampal slices or cultured hippocampal neurons enhanced both DSI19, 42, 43 and DSE43 of CA1 pyramidal neurons. An i.p. injection of 16 mg kg−1 JZL184 prominently inhibited brain MAGL activity and elevated brain 2-AG concentrations.9, 44 Accordingly, we hypothesized that an i.p. injection of 20 mg kg−1 JZL184 could increase CA1 2-AG concentrations, activate presynaptic CB1R to reduce release of GABA and glutamate from CA1 GABAergic and glutamatergic presynaptic terminals. To examine this hypothesis we recorded mIPSCs and mEPSCs of CA1 pyramidal neurons in hippocampal slices prepared 45 min after JZL184 injection. The mIPSCs or mEPSCs are traditionally believed to represent the postsynaptic response to the spontaneous release of single presynaptic GABA- or glutamate-containing vesicles. Therefore, any change in spontaneous vesicle release of presynaptic GABA or glutamate should be reflected as a change in the frequency of mIPSCs or mEPSCs, respectively, whereas any change in postsynaptic sensitivity to GABA or glutamate should be reflected as a change in the amplitude of mIPSCs or mEPSCs, respectively. To confirm mIPSC and mEPSC data, we measured PPF after JZL184 administration. PPF, an enhancement of the synaptic response to the second of two closely spaced action potentials, is believed to reflect the effects of residual calcium from the first action potential on release triggered by the second action potential.45 Both mIPSC/mEPSC and PPF results are not conclusive, because their changes can result from various neuromodulators in addition to 2-AG. It is well established that 2-AG specifically mediates DSI and DSE through presynaptic CB1R activation,19, 42 and therefore changes in DSI or DSE strongly suggest a specific change of GABAergic or glutamatergic synaptic function as a result of altered 2-AG signaling. Overall, our mIPSC/mEPSC, PPF and DSI/DSE data together strongly suggest that 20 but not 5 mg kg−1 JZL184 produce long-lasting disinhibitory effects at CA1 GABAergic synapses through 2-AG activation of presynaptic CB1R without significant effects on 2-AG signaling at CA1 glutamatergic synapses. The exact reason(s) for the discrepancy between previous in vitro data42 and our current in vivo data, in terms of significant 2-AG signaling at glutamatergic synapses following JZL184 application,42 is unknown.
Hippocampal CB1R is distributed in GABAergic and glutamatergic presynaptic terminals46, 47 and in astroglial cells.21 Astrocytes have a highly ramified morphology and occupy at least 50% of brain volume.48 With their unique morphology, astroglial cells are extensively exposed to interstitial fluid and synapses.49 Our findings of astroglial CB1R21 indicate that the interstitial space contains eCB that can directly stimulate astroglial CB1R. A recent study further showed that astroglial cells take up 2-AG secreted from neurons,50 suggesting the existence of interstitial 2-AG flow from neurons to astroglial cells. Thus, an acute blockade of its clearance pathway by JZL184 would produce an acute accumulation of interstitial 2-AG, leading to astroglial CB1R activation. Our recent report21 and current study showed that astroglial CB1R activation by systemic or intra-CA1 injection of synthetic cannabinoids,21 systemic injection of JZL184 (5 or 20 mg kg−1) or intra-CA1 application of 2-AG induced in vivo LTD at CA3-CA1 synapses, which required postsynaptic NR2BR activation and AMPAR endocytosis. Consistent with the finding that LTD maintenance, but not transient synaptic transmission depression, requires new protein synthesis,51, 52 the RNA transcription inhibitor actinomycin-D blocked the late but not the early phase of LTD expression.
In summary, we reported differential antidepressant effects of low- and high-doses of MAGL inhibitors on acutely and chronically stressed mice (Figure 6): (a) a low-dose of MAGL inhibitors produced antidepressant effects likely through astrocyte-mediated LTD at glutamatergic synapses in acutely stressed mice without significant antidepressant effects in chronically stressed mice; (b) a high-dose of MAGL inhibitor treatment before acute stress produced pro-depressant effects through disinhibition of GABAergic synapses, or antidepressant effects through astrocyte-mediated LTD at glutamatergic synapses when GABAergic synaptic disinhibition was blocked; (c) a high but not low-dose of MAGL inhibitors produced rapid and long-lasting antidepressant responses in chronically stressed mice likely through disinhibition of GABAergic synapses. Thus, the high-doses of MAGL inhibitors may be effective in depressed patients with or without significant response to ketamine therapy.
Proposed model for biphasic effects of monoacylglycerol lipase (MAGL) inhibitors on acutely and chronically stressed mice, MAGL inhibition increases 2-AG to trigger the two signaling pathways: (1) activation of GABAergic presynaptic CB1R decreases GABA release to disinhibit postsynaptic neurons, which produces pro-depressant effects on acutely stressed mice (acute stress) or antidepressant effects on chronically stressed mice (chronic stress); (2) 2-AG activates astroglial CB1R, promoting the accumulation of interstitial glutamate to produce postsynaptic NR2BR activation, α-amino-3-hydroxy-5-methyl-isoxazole propionic acid receptors (AMPAR) endocytosis and long-term depression (LTD) expression, which leads to antidepressant effects on acutely stressed mice without significant effects on chronically stressed mice. Thus, a high dose of MAGL inhibitor produces rapid antidepressant effects on chronic-stressed mice through disinhibition of GABAergic synapses.
References
Charney DS, Nestler EJ . Neurobiology of Mental Illness. Oxford University Press: New York, 2005.
Briley M, Moret C . Present and future anxiolytics. IDrugs 2000; 3: 695–699.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163: 28–40.
Rasmussen KG . Has psychiatry tamed the "ketamine tiger?" Considerations on its use for depression and anxiety. Prog Neuropsychopharmacol Biol Psychiatry 2016; 64: 218–224.
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 2013; 170: 1134–1142.
Hill MN, Patel S . Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol Mood Anxiety Disord 2013; 3: 19.
Hillard CJ, Liu QS . Endocannabinoid signaling in the etiology and treatment of major depressive illness. Curr Pharm Des 2014; 20: 3795–3811.
Leite CE, Mocelin CA, Petersen GO, Leal MB, Thiesen FV . Rimonabant: an antagonist drug of the endocannabinoid system for the treatment of obesity. Pharmacol Rep 2009; 61: 217–224.
Blankman JL, Cravatt BF . Chemical probes of endocannabinoid metabolism. Pharmacol Rev 2013; 65: 849–871.
Di Marzo V . Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci 2011; 14: 9–15.
Chevaleyre V, Takahashi KA, Castillo PE . Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 2006; 29: 37–76.
Zoerner AA, Gutzki FM, Batkai S, May M, Rakers C, Engeli S et al. Quantification of endocannabinoids in biological systems by chromatography and mass spectrometry: a comprehensive review from an analytical and biological perspective. Biochim Biophys Acta 2011; 1811: 706–723.
Karatsoreos IN, McEwen BS . Resilience and vulnerability: a neurobiological perspective. F1000Prime Rep 2013; 5: 13.
Pariante CM, Lightman SL . The HPA axis in major depression: classical theories and new developments. Trends Neurosci 2008; 31: 464–468.
Shea A, Walsh C, Macmillan H, Steiner M . Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post-traumatic stress disorder in females. Psychoneuroendocrinology 2005; 30: 162–178.
Roni MA, Rahman S . Effects of lobeline and reboxetine, fluoxetine, or bupropion combination on depression-like behaviors in mice. Pharmacol Biochem Behav 2015; 139: 1–6.
Gourley SL, Taylor JR . Recapitulation and reversal of a persistent depression-like syndrome in rodents. Curr Protoc Neurosci 2009; Chapter 9: 32.
Hill MN, Hillard CJ, Bambico FR, Patel S, Gorzalka BB, Gobbi G . The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends Pharmacol Sci 2009; 30: 484–493.
Zhong P, Wang W, Pan B, Liu X, Zhang Z, Long JZ et al. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology 2014; 39: 1763–1776.
Zhang Z, Wang W, Zhong P, Liu SJ, Long JZ, Zhao L et al. Blockade of 2-arachidonoylglycerol hydrolysis produces antidepressant-like effects and enhances adult hippocampal neurogenesis and synaptic plasticity. Hippocampus 2015; 25: 16–26.
Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 2012; 148: 1039–1050.
Hirrlinger PG, Scheller A, Braun C, Hirrlinger J, Kirchhoff F . Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia 2006; 54: 11–20.
Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 2006; 51: 455–466.
Casanova E, Fehsenfeld S, Mantamadiotis T, Lemberger T, Greiner E, Stewart AF et al. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis 2001; 31: 37–42.
Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 2003; 302: 84–88.
Shimshek DR, Kim J, Hubner MR, Spergel DJ, Buchholz F, Casanova E et al. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis 2002; 32: 19–26.
Jiang W, Zhang Y, Xiao L, Van Cleemput J, Ji S, Bai G et al. Cannabinoid promotes embryonic and adult hippocampus neurogenesis and produces anxiolytic- and antidepression-like effects. J Clin Invest 2005; 115: 3104–3116.
Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry 2008; 63: 353–359.
Steiner MA, Marsicano G, Wotjak CT, Lutz B . Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology 2008; 33: 1165–1170.
Haring M, Grieb M, Monory K, Lutz B, Moreira FA . Cannabinoid CB(1) receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations. Neuropharmacology 2013; 65: 83–89.
Manahan-Vaughan D, Kulla A, Frey JU . Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J Neurosci 2000; 20: 8572–8576.
Kelleher RJ 3rd, Govindarajan A, Tonegawa S . Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron 2004; 44: 59–73.
Kendler KS, Karkowski LM, Prescott CA . Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 1999; 156: 837–841.
Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A . Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry 2011; 70: 479–486.
Aliczki M, Zelena D, Mikics E, Varga ZK, Pinter O, Bakos NV et al. Monoacylglycerol lipase inhibition-induced changes in plasma corticosterone levels, anxiety and locomotor activity in male CD1 mice. Horm Behav 2013; 63: 752–758.
Sciolino NR, Zhou W, Hohmann AG . Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol Res 2011; 64: 226–234.
Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 2010; 13: 1113–1119.
Lomazzo E, Bindila L, Remmers F, Lerner R, Schwitter C, Hoheisel U et al. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacol 2015; 40: 488–501.
Wang M, Hill MN, Zhang L, Gorzalka BB, Hillard CJ, Alger BE . Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol 2012; 26: 56–70.
Hu W, Zhang M, Czeh B, Zhang W, Flugge G . Chronic restraint stress impairs endocannabinoid mediated suppression of GABAergic signaling in the hippocampus of adult male rats. Brain Res Bull 2011; 85: 374–379.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al. Glutamate N-methyl-d-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761.
Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF et al. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances retrograde endocannabinoid signaling. J Pharmacol Exp Ther 2009; 331: 591–597.
Straiker A, Hu SS, Long JZ, Arnold A, Wager-Miller J, Cravatt BF et al. Monoacylglycerol lipase limits the duration of endocannabinoid-mediated depolarization-induced suppression of excitation in autaptic hippocampal neurons. Mol Pharmacol 2009; 76: 1220–1227.
Long JZ, Nomura DK, Cravatt BF . Characterization of monoacylglycerol lipase inhibition reveals differences in central and peripheral endocannabinoid metabolism. Chem Biol 2009; 16: 744–753.
Zucker RS . Short-term synaptic plasticity. Annu Rev Neurosci 1989; 12: 13–31.
Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 2006; 26: 2991–3001.
Bellocchio L, Lafenetre P, Cannich A, Cota D, Puente N, Grandes P et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 2010; 13: 281–283.
Hof PR, Glezer II, Condé F, Flagg RA, Rubin MB, Nimchinsky EA et al. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat 1999; 16: 77–116.
Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M et al. Sleep drives metabolite clearance from the adult brain. Science 2013; 342: 373–377.
Viader A, Blankman JL, Zhong P, Liu X, Schlosburg JE, Joslyn CM et al. Metabolic interplay between astrocytes and neurons regulates endocannabinoid action. Cell Rep 2015; 12: 798–808.
Huber KM, Kayser MS, Bear MF . Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 2000; 288: 1254–1257.
Acknowledgements
This project was supported by operating grants from the Canadian Institutes of Health Research (CIHR) to X. Zhang (MOP123249, MOP46233) and M.N. Hill (MOP119502), the National Natural Science Foundation of China (Grant No. 81571074 to J. Li and Grant No. 81201054 to Y. Wang), the US National Institutes of Health (NIH) to K. Mackie (DA021696 and DA011322), and an equipment grant from the Canadian Foundation for Innovation (CFI) to X. Zhang.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Wang, Y., Gu, N., Duan, T. et al. Monoacylglycerol lipase inhibitors produce pro- or antidepressant responses via hippocampal CA1 GABAergic synapses. Mol Psychiatry 22, 215–226 (2017). https://doi.org/10.1038/mp.2016.22
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.22
This article is cited by
-
How depression and antidepressant drugs affect endocannabinoid system?—review of clinical and preclinical studies
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
The role of endocannabinoids in consolidation, retrieval, reconsolidation, and extinction of fear memory
Pharmacological Reports (2021)
-
Potential application of endocannabinoid system agents in neuropsychiatric and neurodegenerative diseases—focusing on FAAH/MAGL inhibitors
Acta Pharmacologica Sinica (2020)
-
Conformational gating, dynamics and allostery in human monoacylglycerol lipase
Scientific Reports (2020)
-
Integrating endocannabinoid signaling in the regulation of anxiety and depression
Acta Pharmacologica Sinica (2019)