Abstract
One hundred years after its conceptual definition as ‘Dementia Praecox’ by Emil Kraepelin, schizophrenia is still a serious psychiatric illness that affects young adults and leads to disability in at least half of patients. The key treatment issue is partial or non-response, especially of negative symptoms. The illness is also associated with different degrees of cognitive dysfunction, particularly in verbal and working memory; the resulting functional impairment may lead to unemployment and an inability to maintain stable relationships. Patients’ cognitive dysfunction led Kraepelin to the assumption that schizophrenia is a form of juvenile dementia caused by a degenerative process of the human brain. Postmortem studies and a plethora of imaging studies do not support the notion of a degenerative process, but such a process is supported by the recently published, largest genome-wide association study on schizophrenia. More than a 100 hits were described, converging on pathways that have a significant role in dopamine metabolism in immune modulation, calcium signalling and synaptic plasticity. This review suggests that research should focus on animal models based on risk genes like transcription factor 4 and study the effects of exposure to environmental stressors relevant for schizophrenia. The use of relevant end points like pre-pulse inhibition or cognitive dysfunction will allow us to gain an understanding of the molecular pathways in schizophrenia and consequently result in improved treatment options, especially for the disabling aspects of this illness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kraepelin E . Psychiatrie: ein Lehrbuch für Studirende und Aertze [Psychiatry: A Textbook for Students and Doctors], 6th edn, JA Barth: Lepizig, Germany, 1899.
Alzheimer A . Neuere Arbeiten über die Dementia senilis [recent works on senile dementia]. Monatsschrift für Psychiatrie und Neurologie 1893; 3: 101–115.
Kraepelin E . Lehrbuch der Psychiatrie [Textbook of Psychiatry], 8th edn, JA Barth: Leipzig, Germany, 1913.
Bleuler E . Die schizophrenen Geistesstörungen im Lichte langjähriger Kranken- und Familiengeschichten. Thieme: Stuttgart, Germany, 1908.
Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl 1992; 20: 1–97.
Jablensky A . Epidemiology of schizophrenia: the global burden of disease and disability. Eur Arch Pschiatry Clin Neurosci 2000; 250: 274–285.
Häfner H, an der Heiden W . Course and outcome of schizophrenia. In: Hirsch R, Weinberger D (eds) Schizophrenia, 2nd edn, Blackwell: Malden, MA, USA, pp 101–139 2003.
Marengo J . Classifying the courses of schizophrenia. Schizophr Bull 1994; 20: 519–536.
Lambert M, Karow A, Leucht S, Schimmelmann BG, Naber D . Remission in schizophrenia: validity, frequency, predictors, and patients' perspective 5 years later. Dialogies Clin Neurosci 2010; 12: 393–407.
Hoff AL, Svetina C, Shields G, Stewart J, DeLisi LE . Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr Res 2005; 78: 27–34.
Green MF . What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153: 321–330.
Silverstein SM, Schenkel LS, Valone C, Nuernberger SW . Cognitive deficits and psychiatric rehabilitation outcomes in schizophrenia. Psychiatr Q 1998; 69: 169–191.
Goff DC, Hill M, Barch D . The treatment of cognitive impairment in schizophrenia. Pharmacol Biochem Behav 2010; 99: 245–253.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–427.
Walters JT, Rujescu D, Franke B, Giegling I, Vásquez AA, Hargreaves A . The role of the major histocompatibility complex region in cognition and brain structure: a schizophrenia GWAS follow-up. Am J Psychiatry 2013; 170: 877–885.
Corvin A, Morris DW . Genome-wide association studies: findings at the major histocompatibility complex locus in psychosis. Biol Psychiatry 2014; 75: 276–283.
McAllister AK . Major histocompatibility complex I in brain development and schizophrenia. Biol Psychiatry 2014; 75: 262–268.
Pribiag H, Stellwagen D . Neuroimmune regulation of homeostatic synaptic plasticity. Neuropharmacology 2014; 78: 13–22.
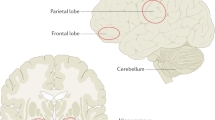
Haijma SV, Van Haren N, Cahn W, Koolschijn PC, Hulshoff Pol HE, Kahn RS . Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull 2013; 39: 1129–1138.
Ellison-Wright I, Bullmore E . Anatomy of bipolardisorder and schizophrenia: a meta-analysis. Schizophr Res 2010; 117: 1–12.
De Peri L, Crescini A, Deste G, Fusar-Poli P, Sacchetti E, Vita A . Brain structural abnormalities at the onset of schizophrenia and bipolar disorder: a meta-analysis of controlled magnetic resonance imaging studies. Curr Pharm Des 2012; 18: 486–494.
Ellison-Wright I, Bullmore E . Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 2009; 108: 3–10.
Hasan A, Wobrock T, Falkai P, Schneider-Axmann T, Guse B, Backens M et al. Hippocampal integrity and neurocognition in first-episode schizophrenia: a multidimensional study. World J Biol Psychiatry 2014; 15: 188–199.
Kühn S, Gallinat J . Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull 2013; 39: 358–365.
Adriano F, Caltagirone C, Spalletta G . Hippocampal volume reduction in first-episode and chornic schizophrenia: a review and meta-analysis. Neuroscientist 2012; 18: 180–200.
Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ . Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev 2012; 36: 1342–1356.
Bogerts B, Falkai P, Haupts M, Greve B, Ernst S, Tapernon-Franz U et al. Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophr Res 1990; 3: 295–301.
Bogerts B, Meertz E, Schonfeldt-Bausch R . Basal ganglia and limbic system pathology in schizophrenia. A morphometric study of brain volume and shrinkage. Arch Gen Psychiatry 1985; 42: 784–791.
Wood SJ, Kennedy D, Phillips LJ, Seal ML, Yücel M, Nelson B . Hippocampal pathology in individuals at ultra-high risk for psychosis: a multi-modal magnetic resonance study. Neuroimage 2010; 52: 62–68.
Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL et al. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 2009; 117: 395–407.
Falkai P, Bogerts B . Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 1986; 236: 154–161.
Stocum DL . Regenerative Biology and Medicine. Academic Press: San Diego, CA, USA, 2010.
Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, Lesch K-P . Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 2006; 11: 514–522.
Toro CT, Deakin JFW . Adult neurogenesis and schizophrenia: a window on abnormal early brain development. Schizophr Res 2007; 90: 1–14.
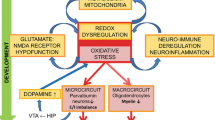
Schmitt A, Hasan A, Gruber O, Falkai P . Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci 2011; 261: S150–S154.
Morrison BM, Lee Y, Rothstein JD . Oligodendroglia: metabolic supporters of axons. Trends Cell Biol 2013; 23: 644–651.
Sawada K, Barr AM, Nakamura M, Arima K, Young CE, Dwork AJ . Hippocampal complexin proteins and cognitive dysfunction in schizophrenia. Arch Gen Psychiatry 2005; 62: 263–272.
Schmitt A, Leonardi-Essmann F, Durrenberger PF, Wichert SP, Spanagel R, Arzberger T et al. Structural synaptic elements are differentially regulated in superior temporal cortex of schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 2012; 262: 565–577.
Anderson KK, Voineskos A, Mulsant BH, George TP, McKenzie KJ . The role of untreated psychosis in neurodegeneration: a review of hypothesized mechanosms of neurotoxicity in first-episode psychosis. Can J Psychiatry 2014; 59: 513–517.
Pino O, Guilera G, Gómez-Benito J, Najas-Garcia A, Rufián S, Rojo E . Neurodevelopment of neurodegeneration: reviews of theories of schizophrenia. Actas Esp Psiquiatr 2014; 42: 185–195.
Brzózka MM, Radyushkin R, Wichert SP, Ehrenreich H, Rossner MJ . Cognitive and sensorimotor gating impairments in transgenic mice overexpressing the schizophrenia susceptibility gene Tcf4 in the forebrain. Biol Psychiatry 2010; 68: 33–40.
Brzózka MM, Rossner MJ . Deficits in trace fear memory in a mouse model of the schizophrenia risk gene TCF4. Behav Brain Res 2013; 237: 348–356.
Quednow BB, Brzozka MM, Rossner MJ . Transcription factor 4 (TCF4) and schizophrenia: integrating the animal and the human perspective. Cell Mol Life Sci 2014; 71: 2815–2835.
Wirgenes KV, Sonderby IE, Haukvik UK, Mattingsdal M, Tesil M, Athanasiu L et al. TCF4 sequence variants and mRNA levels are associated with neurodevelopmental characteristics in psychotic disorders. Transl Psychiatry 2012; 2: e112.
Brennand KJ, Simone A, Jou J, Belboin-Burkhart C, Tran N, Sangar S et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 2011; 473: 221–225.
Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM et al. An in vivo correlated of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA 2007; 104: 5638–5643.
Erickson KI, Voss MW, Prakash RS, Basek C, Szabo A, Chaddock L et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011; 108: 3017–3022.
Biedermann S, Fuss J, Zheng L, Sartorius A, Falfan-Melgoza C, Demirakca T et al. In vivo voxel based morphometry: detection of increased hippocampal volume and decreased glutamate levels in exercising mice. Neuroimage 2012; 61: 1206–1212.
van Praag H, Christie BR, Sejnowski TJ, Gage FH . Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA 1999; 96: 13427–13431.
Naylor AS, Bull C, Nilsson MKL, Zhu C, Björk-Eriksson T, Eriksson PS et al. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young muse brain. Proc Natl Acad Sci USA 2008; 105: 14632–14637.
Morroni F, Kitazawa M, Drago D, Cheng D, Medeiros R, LaFerla FM . Repeated physical training and environmental enrichment induce neurogenesis and synaptogenesis following neuronal injury in an inducable mouse model. J Behav Brain Sci 2011; 1: 199–209.
Hasan A, Wobrock T, Rajji T, Malchow B, Daskalakis ZJ . Modulating neural plasticity with non-invasive brain stimulation in schizophrenia. Eur Arch Psychiatry Clin Neurosci 2013; 263: 621–631.
Frantseva MV, Fitzgerald PB, Chen R, Möller B, Daigle M, Daskalakis ZJ . Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cereb Cortex 2008; 18: 990–996.
Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R . Dysfunctional neural plasticity in patients with schizophrenia. Arch Gen Psychiatry 2008; 65: 378–385.
Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O et al. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav Brain Res 2011; 224: 15–22.
Fitzgerald PB, Brown TL, Marston NA, Oxley T, De Castella A, Daskalakis ZJ et al. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophr Res 2004; 71: 17–26.
Hasan A, Nitsche MA, Herrmann M, Schneider-Axmann T, Marshall L, Gruber O et al. Impaired long-term depression in schizophrenia: a cathodal tDCS pilot study. Brain Stimul 2012; 5: 475–483.
Strube W, Nitsche MA, Wobrock T, Bunse T, Rein B, Herrmann M et al. BDNF-Val66Met-polymorphism imoact on cortical plasticity in schizophrenia patients: a proof-of-concept study. Int J Neuropsychopharmacol 2014; 18, e-pub ahead of print 31 October 2014; doi:10.1093/ijnp/pyu040.
Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry 2010; 67: 133–143.
Schmitt A, Malchow B, Hasan A, Falkai P . The impact of environmental factors in severe psychiatric disorders. Front Neurosci 2014; 8: 19.
Hasan A, Mitchell A, Schneider A, Halene T, Akbarian S . Epigenetic dysregulation in schizophrenia: molecular and clinical aspects of histone deacetylase inhibitors. Eur Arch Psychiatry Clin Neurosci 2013; 263: 273–284.
Wright C, Turner JA, Calhoun VD, Perrone-Bizzozero N . Potential impact of miR-137 and its targets in schizophrenia. Front Genet 2013; 4: 58.
Peter CJ, Akbarian S . Balancing histone methylation activities in psychiatric disorders. Trends Mol Med 2011; 17: 372–379.
Chase KA, Gavin DP, Guidotti A, Sharma RP . Histone methylation at H3K9: evidence for a restrictive epigenome in schizophrenia. Schizophr Res 2013; 149: 15–20.
Kato T, Iwamoto K . Comprehensive DNA methylation and hydroxymethylation analysis in the human brain and its implication in mental disorders. Neuropharmacology 2014; 80: 133–139.
Wockner LF, Noble EP, Lawford BR, Young RM, Morris CP, Whitehall VL et al. Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients. Transl Psychiatry 2014; 4: e339.
Fischer A, Sananbenesi F, Mungenast A, Tsai L-H . Targeting the correct HDAC(s) to treat cognitive disorders. Trends Pharmacol Sci 2010; 31: 605–617.
Acknowledgements
This work is supported by the Deutsche Forschungsgemeinschaft via the Clinical Research Group 241 ‘Genotype–phenotype relationships and neurobiology of the longitudinal course of psychosis’ (http://www.kfo241.de; grant number FA 241/16-1, SCHU 1603/5-1 and RO 4076/1-1). The authors thank Jacquie Klesing, Board-certified Editor in the Life Sciences, for editing assistance with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Falkai, P., Rossner, M., Schulze, T. et al. Kraepelin revisited: schizophrenia from degeneration to failed regeneration. Mol Psychiatry 20, 671–676 (2015). https://doi.org/10.1038/mp.2015.35
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.35
This article is cited by
-
Cellular pathology in the limbic system in schizophrenia
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Blood–brain barrier dysfunction and folate and vitamin B12 levels in first-episode schizophrenia-spectrum psychosis: a retrospective chart review
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Optical coherence tomography reveals retinal thinning in schizophrenia spectrum disorders
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
Spironolactone alleviates schizophrenia-related reversal learning in Tcf4 transgenic mice subjected to social defeat
Schizophrenia (2022)
-
Abnormal corneal nerve morphology and brain volume in patients with schizophrenia
Scientific Reports (2022)