Abstract
Major depressive disorder is an extremely debilitating condition affecting millions of people worldwide. Nevertheless, currently available antidepressant medications still have important limitations, such as a low response rate and a time lag for treatment response that represent a significant problem when dealing with individuals who are vulnerable and prone to self-harm. Recent clinical trials have shown that the N-methyl-D-aspartate receptor antagonist, ketamine, can induce an antidepressant response within hours, which lasts up to 2 weeks, and is effective even in treatment-resistant patients. Nonetheless, its use is limited due to its psychotomimetic and addictive properties. Understanding the molecular pathways through which ketamine exerts its antidepressant effects would help in the developing of novel antidepressant agents that do not evoke the same negative side effects of this drug. This review focuses specifically on the effects of ketamine on three molecular mechanisms that are relevant to depression: synaptogenesis, immunomodulation and regulation of glycogen synthase kinase-3 activity.
Similar content being viewed by others
Introduction
Recent clinical research has provided evidence demonstrating that low-dose intravenous infusions of ketamine, a drug originally developed as an anaesthetic,1 can improve depressive symptoms within hours in subjects with treatment-resistant depression.2 Interestingly, the effects have been shown to last from a couple of days up to several weeks.3, 4, 5 This is a crucial step forward in the treatment of depression, among the greatest challenges that modern medicine has ever been forced to face, thought to affect up to 350 million people worldwide.6 The antidepressant medications available today exhibit low rates of treatment response, with only one in three people responding to their first prescribed medication and two in three people responding after trying numerous alternatives.7 More importantly, therapeutic effects display a response lag time of several weeks, a significant problem in those individuals who are particularly vulnerable to self-harm and suicide. For these reasons, there is a pressing need to identify novel antidepressant drugs that are fast acting and show better rates of response.
Despite the promising rapid antidepressant action, ketamine has psychotomimetic and addictive properties that limit its potential widespread use as a fast-acting antidepressant drug. Indeed, ketamine has been shown to induce psychosis in healthy subjects8 and to exacerbate psychotic symptoms in individuals affected by schizophrenia,9 and has also been abused as a ‘club drug’.10 Elucidating the molecular pathways via which ketamine mediates its antidepressant effects would facilitate the development of other pharmacological agents with similar beneficiary properties but without the unwanted side effects. A recent review11 has discussed the putative synaptic actions of the drug. Here we focus on three specific molecular mechanisms that are potentially involved in the antidepressant action of ketamine: increased neuroplasticity and synaptogenesis via enhancement of glutamatergic signaling, changes in immune function and (more preliminary) regulation of glycogen synthase kinase-3 (GSK-3) activity.
Ketamine acts via synaptogenesis promotion through enhanced glutamatergic signaling
Ketamine is classified pharmacologically as an N-methyl-D-aspartate (NMDA) receptor antagonist. The first indication that the NMDA receptor may be a useful target for antidepressant treatment came from observations that the anti-tuberculosis drug, cycloserine, a partial agonist of the glycine site of the NMDA receptor, improved mood in those tuberculosis-affected patients who were also depressed.12 It subsequently took more than 30 years to develop the hypothesis that compounds altering NMDA function could have antidepressant properties13 and therefore that glutamate, its main ligand, may be involved in the pathophysiology of depression. The NMDA receptor is a specific type of ionotropic glutamate receptor. However, it has been suggested that other glutamatergic receptors may be involved in the action of ketamine. Indeed, when glutamate (L-glutamic acid), the major excitatory neurotransmitter in the nervous system, is released from presynaptic neurons, it can interact with different postsynaptic receptors: kainite, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), and NMDA. Several findings support the involvement of both NMDA and AMPA receptors in the pathophysiology of major depression disorder (MDD) and in the mechanisms of action of antidepressants.14, 15, 16, 17 Ketamine is able to increase extracellular glutamate levels in the prefrontal cortex (PFC) by inhibiting NMDA receptor currents on GABAergic interneurones, in turn disinhibiting glutamate transmission.18, 19 Additionally, ketamine can enhance AMPA receptor throughput.20 In particular, an increase in AMPA/NMDA receptor density ratio has been observed in the hippocampus of rats after ketamine treatment.21 Indeed, an enhanced glutamatergic activity transduced through AMPA receptors rather than NMDA receptors may be responsible for mediating the increased synaptic potentiation and activation of early neuroplastic genes observed upon exposure to the drug15 (described below in further detail). Of note, treatment with NBQX, an AMPA receptor antagonist, has been shown to inhibit the antidepressant effects of ketamine in animal models of depression.22, 23 Both AMPA and NMDA receptors are important in long-term potentiation and long-term depression, the two main neurobiological mechanisms which are responsible for mediating activity-dependent synaptic plasticity and re-modeling. Long-term potentiation has primarily been shown to induce dendritic spine growth, and enlargement of pre-existing spines and of the associated post-synaptic density proteins,24 all of which are observed upon ketamine exposure.25 Additionally, ketamine inhibits spontaneous NMDA mini-excitatory post-synaptic currents caused by spontaneous glutamate release at rest.26 Interestingly, brain post mortem studies have described increased glutamate levels in individuals with mood disorders,27 while decreased hippocampal NMDA receptors have been described in bipolar patients.28
Ketamine has also been shown to reverse the dendritic atrophy caused by chronic unpredictable stress exposure, a paradigm widely used to induce depression-like behavior in rodents. In such a model, a single dose of ketamine increased the number of spines on the apical dendrites of PFC layer V pyramidal neurons and also their function, as demonstrated by increases in serotonin- and hypocretin-induced excitatory post-synaptic currents. These changes occurred 2 h after administration of the drug and were sustained for up to 7 days, a time course comparable to that reported in clinical trials.5 Concomitantly, the reduced interest in both sucrose and food that followed chronic unpredictable stress was completely abolished.29
Ketamine action on neurotrophic factors: brain-derived neurotrophic factor (BDNF)
Ketamine has been shown to regulate levels of the neurotrophin BDNF.30, 31 BDNF has a central role in the neurotrophic theory of depression, which proposes that stress-related reduction in neurotrophic support, leading to the degeneration of limbic structures and, in particular, of the PFC and hippocampus, represents an important factor underlying the pathogenesis of depression. More specifically, reductions in BDNF, and of its high affinity receptor TrkB, have been found both in the blood and the brains of patients affected by mood disorders; conversely, antidepressant drugs have shown to act, at least in part, through a potentiation of BDNF expression and its signaling.32, 33, 34, 35, 36 BDNF has also been associated with the process of neurogenesis, thought to be impaired in stress and increased by antidepressants.37, 38, 39 Additionally, BDNF has been linked to synaptic re-modeling, being able to both induce and be induced by long-term potentiation.40, 41 Animal models have shown that ketamine administration reduced immobility in the forced swim test, and this behavioral effect was coupled with increased BDNF protein levels in the hippocampus.30 Furthermore, circulating BDNF levels were increased after ketamine administration in treatment-resistant MDD patients concomitant with mood improvement.42 Interestingly, AMPA receptor agonists have shown similar actions to ketamine. For example, treatment of primary neuronal cultures and hippocampal slices with the agonist CX614 led to increased dendritic protein synthesis, mediated by BDNF secretion and TrkB receptor activation.43 Additionally, the novel AMPA receptor potentiator LY392098 has been shown to increase the expression of BDNF in primary neuronal cultures.44
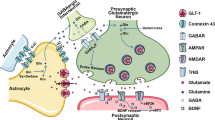
Several mechanisms have been proposed to explain the modulation of BDNF induced by ketamine, as schematized in Figure 1. Ketamine-mediated suppression of resting NMDA receptor activity can lead to inhibition of eukaryotic elongation factor 2 (eEF2) kinase and subsequently to a dephosphorylation of eEF2, with a concomitant augmentation of BDNF synthesis.45 Alternatively, or additionally, the resultant depolarization from AMPA receptor activity can activate voltage-dependant calcium channels, allowing calcium influx and exocytosis of BDNF, which can then activate TrkB receptors, in turn setting off an intracellular signaling cascade that includes phosphorylation and thus activation of Akt (also known as protein kinase B) and extracellular signal-regulated protein kinase (ERK). Both Akt and ERK are involved in the regulation of synaptic protein synthesis (described in detail in the next section).44, 46 Of note, it has also been proposed that the upregulation in BDNF and synaptic protein expression could be due to the de-suppression of translation and not to activity-dependent BDNF release or intracellular TrkB signaling.47
Schematic representation of mechanisms underlying ketamine action. Ketamine-mediated suppression of N-methyl-D-aspartate receptor (NMDA-R) activity leads to inhibition of eukaryotic elongation factor 2 (eEF2) kinase and a subsequent dephosphorylation of eEF2, with a concomitant augmentation of brain-derived neurotrophic factor (BDNF) synthesis. The depolarization from α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPA-R) activity activates voltage-dependant calcium channels (VDCCs), allowing calcium influx and exocytosis of BDNF, which can then activate TrkB receptors, in turn activating Akt and extracellular signal-regulated protein kinase (ERK). Akt and ERK activate mammalian target of rapamycin (mTOR), which enables the translation of synaptic protein by activating p70S6 kinase and inhibiting 4E binding proteins (4E-BPs). Glycogen synthase kinase-3 can be released from its inhibition of BDNF expression through phosphorylation by Akt or p70S6K. See text for additional details.
Synaptic-relevant signaling pathways underlying ketamine-induced synaptogenesis: mammalian target of rapamycin (mTOR)
As the production of synaptic proteins that are important for neuroplasticity is one of the potentially critical steps for ketamine action,46 attention has recently turned to those enzymes involved in their synthesis. One of these enzymes is the serine/threonine kinase mTOR, whose activation is essential in regulating the expression of several proteins involved in synaptic plasticity.11 Interestingly, reduced synaptic proteins in conjunction with reduced mTOR signaling have been found in the PFC of depressed subjects, highlighting the importance of mTOR.48 Therefore, it is worth describing briefly the signaling cascades that both activate and are activated by it (shown in Figure 1). mTOR can be phosphorylated by several kinases, including Akt and ERK, which are both triggered by neurotrophic factor signaling cascades (as described above). mTOR then enables the translation of synaptic protein by activating p70S6 kinase and inhibiting the inhibitory 4E binding proteins (4E-BPs).49, 50 Importantly, administration of ketamine to rats has shown a rapid induction of phosphorylation of mTOR, p70S6 kinase and 4E-BP1 in synaptoneurosome PFC preparations. This induction was accompanied by an upregulation of Arc, GluR1, PSD95 and synapsin I, which are all markers of synaptic plasticity and found to be decreased upon exposure to stress in the learned helplessness paradigm of depression.25 The importance of mTOR is emphasized by results obtained on pre-treatment with the inhibitor rapamycin, which blocks cell-cycle progression and prevents p70S6 kinase activation.51, 52 This inhibition completely repressed the behavioral antidepressant effects of ketamine when tested in both the forced swim test and learned helplessness paradigms.25 In line with this behavioral effect, rapamycin blocked ketamine induction of layer V pyramidal PFC neuron spine number and function, as well as the expression of synaptic proteins. Furthermore, co-treatment with ketamine and the AMPA receptor antagonist, NBQX, completely block 4E-PB1, p70S6k, mTOR, ERK and Akt phosphorylation. The involvement of mTOR or eEF2, however, is far from clear. A recent study in female rats showed no changes in the phosphorylation of either of them as mediating the response to ketamine,53 suggesting that at least some of the underlying mechanisms may be sex-specific. It is generally agreed, though, that ketamine-induced synaptogenesis appears to be a result of NMDA receptor blockade at rest, which leads to the de-suppression of translation of rapid dendritic proteins and BDNF. Of relevance here, BDNF-evoked protein translation in neuronal dendrites has been reported to be attenuated by both rapamycin and small interfering RNAs specific for mTOR.54 It is important to mention that mTOR can form two complexes—complex 1 (mTORC1) and complex 2 (mTORC2), defined by the presence of regulatory-associated protein of mTOR or rapamycin-independent companion of mTOR, respectively.55, 56 Although publications examining the effects of ketamine on synaptogenesis have not yet differentiated between these two complexes, observations with the inhibitor rapamycin would suggest that studies have indeed focused on mTORC1.51, 52 It is theoretically possible, though, that mTORC2 is also involved in the action of ketamine as it activates Akt and has downstream effects that are important in the organization of the actin cytoskeleton.57 However, the above-mentioned ability of a brief pre-treatment with rapamycin to abolish the action of ketamine25 strongly suggests that mTORC1 is the principal effector, given that rapamycin robustly and rapidly inhibits mTORC1 and only partially and slowly acts on mTORC2.55 To further understand both complexes, more specific blockers would have to be employed.
Ketamine immunomodulatory actions
Several reports have shown that ketamine can limit and even prevent inflammation.58 High levels of inflammation have been reported to be important in depression59, 60 and appear to influence treatment response.61, 62 We will describe two main mechanisms that have been proposed for ketamine: a direct action on inflammatory cytokines and regulators and an involvement in the kynurenine pathway.
A recent meta-analysis has shown that ketamine administration before or during surgery significantly inhibits the early post-operative interleukin (IL)-6 inflammatory response in patients.63 Additionally, ketamine is able to suppress lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α, IL-6 and IL-8 production in the human whole blood.64 Similar results were observed upon ketamine treatment of rats, showing an attenuation of both the increase in TNF-α as well as the increase in the ratio of IL-6 to IL-10 following an Escherichia coli endotoxin challenge.65 Further tests in animals have shown suppression of IL-6 and TNF-α,66 as well as nitric oxide,67 subsequent to an LPS insult. A series of experiments have shed light into some of the mechanisms underlying these changes in inflammatory markers. For example, studies in a human monocytic cell line indicated that the immunoinhibitory effects of ketamine appear to be caused by inhibition of activation of the transcription factor nuclear factor-kappa B (NF-κB).68 Interestingly, ketamine has also shown to cause inhibition of the expression of the Toll-like receptor (TLR) 4, as well as attenuation of the phosphorylation of p65, one of the subunits of NF-κB, in astrocytes challenged by LPS.69 In addition, ketamine-induced inhibition of lipoteichoic acid-induced TNF-α and IL-6 was also shown to be mediated by inhibition of translocation and transactivation of NF-κB.70 These effects occurred through downregulation of TLR2-mediated phosphorylation of ERK1/2. Additionally, ketamine has displayed inhibition of inflammation via upregulation of the inducible heme oxygenase-1, which can provide cellular protection by exerting antioxidative effects.71
Ketamine can act through an involvement in the kynurenine pathway. As mentioned before, ketamine has proven to be effective in reducing suicidal symptoms,3, 72, 73 and recent evidence points toward a low grade of inflammation in the brain of suicide victims.74, 75 In particular, significantly elevated levels of quinolinic acid (QUIN), associated with higher levels of IL-6, have been reported in the cerebrospinal fluid of suicide attempters.76 This increase in QUIN, an NMDA receptor agonist, correlated with the scores on the Suicide Intent Scale. QUIN is an end product of tryptophan metabolism, in which the enzyme indoleamine 2,3-dioxygenase (IDO), induced by cytokines,77 directs tryptophan away from serotonin and toward kynurenine. Further metabolism of kynurenine can then lead to QUIN and also, or alternatively, to kynurenic acid (KYNA), an NMDA receptor antagonist. Interestingly, suicidal attempters showed no changes in KYNA levels. The increased QUIN/KYNA ratio supports the hypothesis of an overall NMDA receptor stimulation, suggesting that changes in glutamatergic neurotransmission could be specifically linked to suicidality. Further support for the participation of the kynurenine pathway comes from a recent study in mice. Exposure to ketamine immediately before or after administration of LPS abrogated the development of LPS-induced depressive-like behavior (known to occur via activation of IDO78), without altering the LPS-induced sickness. Interestingly, ketamine was effective once inflammation and IDO activation had developed. The role of NMDA receptor antagonism by ketamine was additionally confirmed when mice pre-treated with the AMPA receptor antagonist NBQX displayed a restoration of the depressive-like phenotype upon exposure to both LPS and ketamine.79 A complete evaluation of the regulation of all enzymes within the kynurenine pathway upon ketamine administration will be clearly of great interest.
Ketamine, GSK-3 and circadian rhythm abnormalities
Administration of ketamine to mice has been shown to inhibit brain GSK-3,80 a kinase that, interestingly, is also a target of mood-stabilizing agents.81 The inhibition observed upon ketamine administration occurs via an increase in serine-phosphorylation of both the α and β isoforms of the enzyme.82 Indeed, animals with a knock-in mutation that blocks GSK-3 phosphorylation did not respond to ketamine treatment in the learned helplessness paradigm,80 demonstrating that ketamine-induced phosphorylation of GSK-3 is required for its antidepressant properties. GSK-3 is, in fact, involved in the same pathway as mTOR, being phosphorylated and therefore inactivated by both Akt83 and p70S6K,84 as seen in Figure 1. Furthermore, inhibition of GSK-3 by either short interference RNA or through pharmacological agents has also been shown to increase BDNF, thus implicating GSK-3 in synaptogenesis.85 However, the full role of GSK-3 is still not clearly understood. For example, the GSK-3 inhibitor SB216763 was not able to produce a long-lasting antidepressant action in mice subjected to a chronic mild stress paradigm, when compared with ketamine administration.86 Further research in this area is warranted.
Other possible mechanisms involving GSK-3 have been described. Ketamine, through a mechanism that involves this kinase, is able to influence the circadian molecular machinery. Abnormalities in circadian rhythms have been associated with the pathophysiology of depression. Conversely, therapies like sleep deprivation, which are capable of phase-shifting behavioral and physiological rhythms, have been shown to induce rapid improvement in subsets of depressed patients.87 Ketamine can modulate the expression of several genes involved in the circadian rhythm. In particular, it has been shown that acute exposure of animal neuroblastoma cells to ketamine led to a reduction in the amplitude of circadian transcription of the genes brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1), period 2 (Per2) and cryptochrom 1.88 Furthermore, ketamine altered the recruitment of the circadian locomotor output cycles kaput (CLOCK):BMAL1 complex on circadian promoters. Interestingly, the ketamine-induced repression of CLOCK:BMAL1 was reduced after treatment with the GSK-3 inhibitor SB216763. Of note, mTOR, mentioned above as being required for the antidepressant effects of ketamine, has been implicated in having a key role in the entrainment of the suprachiasmatic nucleus (the central regulator of circadian rhythms in mammals) to light. mTOR is also involved in the resetting of the circadian clock by regulating the synthesis of the core circadian gene protein, PER1 and PER2.89 Interestingly, high doses of ketamine have been shown to block light-induced phase shifts in locomotor activity when administered to hamsters,90 further suggesting that circadian rhythm regulation could have an important role underlying the mode of action of the drug.
Conclusion and future directions
Here we have described evidence supporting the notion that ketamine exerts antidepressant properties mainly via modulation of synaptogenesis and inflammation (and possibly GSK-3). In particular, ketamine is able to improve synaptogenesis by acting on NMDA and AMPA receptors, and probably through stimulation of mTOR activity, which in turn triggers the translation of synaptic proteins required for neuronal plasticity. Additionally, ketamine potentiates BDNF/TrkB signaling. Moreover, ketamine is able to reduce inflammation, an effect that could occur via inhibition of NF-κB or by preventing IDO activation and the possible concomitant shift of tryptophan metabolism toward neurodegenerative metabolites. Finally, GSK-3 appears to have an important role, as its inhibition is required for ketamine to convey its antidepressant effects. Of interest, recent evidence has shown that the antidepressant effects of ketamine were completely abolished when female rats were ovariectomized, and restored upon oestrogen and progesterone supplementation, suggesting a critical role for gonadal hormones.53 This may be clinically relevant, as ketamine has already shown sex-specific differences, both in rat models of analgesia and catalepsy91 and in human studies looking at amnestic effects.92 Additional work in this area is therefore pertinent.
The growing understanding of the mode of action of ketamine has triggered an increased interest by pharmaceutical companies in the development of novel and more effective antidepressant drugs. Indeed, traxoprodil, an NR2B subtype selective NMDA antagonist,93 and GLYX-13, an NMDA receptor glycine-site functional partial agonist,94 are some of those. Interestingly, GLYX-13 is currently in a phase II clinical development programme for treatment-resistant depression. Given the evidence that ketamine is effective in cases of suicidal ideation, improved agents will clearly help to deal with this medical emergency. The hope that a new type of fast acting antidepressants would bring to the growing numbers of MDD patients and their worried families clearly warrants further funding and increased efforts from the scientific community and pharmaceutical companies.
References
Lanning CF, Harmel MH . Ketamine anesthesia. Annu Rev Med 1975; 26: 137–141.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354.
Price RB, Nock MK, Charney DS, Mathew SJ . Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 2009; 66: 522–526.
Thakurta RG, Ray P, Kanji D, Das R, Bisui B, Singh OP . Rapid antidepressant response with ketamine: is it the solution to resistant depression? Indian J Psychol Med 2012; 34: 56–60.
Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864.
WHO. Depression: A Global Crisis. World Health Organisation—World Federation for Mental Health, 2012.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry 2006; 163: 28–40.
Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D et al. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 1996; 14: 301–307.
Lahti AC, Koffel B, LaPorte D, Tamminga CA . Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology 1995; 13: 9–19.
De Luca MT, Meringolo M, Spagnolo PA, Badiani A . The role of setting for ketamine abuse: clinical and preclinical evidence. Rev Neurosci 2012; 23: 769–780.
Dwyer JM, Duman RS . Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry 73: 1189–1198.
Crane GE . Cyloserine as an antidepressant agent. Am J Psychiatry 1959; 115: 1025–1026.
Trullas R, Skolnick P . Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol 1990; 185: 1–10.
Javitt DC . Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry 2004; 9: 984–997, 979.
Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA Jr. . Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther 2009; 123: 143–150.
Hashimoto K . Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev 2009; 61: 105–123.
Hashimoto K . The role of glutamate on the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1558–1568.
Moghaddam B, Adams B, Verma A, Daly D . Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17: 2921–2927.
Homayoun H, Moghaddam B . NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 2007; 27: 11496–11500.
Zarate CA Jr., Manji HK . The role of AMPA receptor modulation in the treatment of neuropsychiatric diseases. Exp Neurol 2008; 211: 7–10.
Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L . Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience 2012; 213: 72–80.
Koike H, Iijima M, Chaki S . Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 2011; 224: 107–111.
Maeng S, Zarate CA Jr. . The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep 2007; 9: 467–474.
Malenka RC, Bear MF . LTP and LTD: an embarrassment of riches. Neuron 2004; 44: 5–21.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al. NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature 2011; 475: 91–95.
Hashimoto K, Sawa A, Iyo M . Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry 2007; 62: 1310–1316.
Scarr E, Pavey G, Sundram S, MacKinnon A, Dean B . Decreased hippocampal NMDA, but not kainate or AMPA receptors in bipolar disorder. Bipolar Disord 2003; 5: 257–264.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761.
Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC et al. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 140–144.
Lindén AM, Väisänen J, Lakso M, Nawa H, Wong G, Castrén E . Expression of neurotrophins BDNF and NT-3, and their receptors in rat brain after administration of antipsychotic and psychotrophic agents. J Mol Neuroscie 2000; 14: 27–37.
Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT . Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 2001; 50: 260–265.
Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G . Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry 2005; 57: 1068–1072.
Monteleone P, Serritella C, Martiadis V, Maj M . Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disord 2008; 10: 95–100.
Sen S, Duman R, Sanacora G . Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 2008; 64: 527–532.
Duman RS, Li N . A neurotrophic hypothesis of depression: role of synaptogenesis in the actions of NMDA receptor antagonists. Philos Trans R Soc Lond B Biol Sci 2012; 367: 2475–2484.
Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E et al. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology 2013; 38: 872–883.
Anacker C, Cattaneo A, Musaelyan K, Zunszain PA, Horowitz M, Molteni R et al. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc Natl Acad Sci USA 2013; 110: 8708–8713.
Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S et al. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry 2011; 16: 738–750.
Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A . Postsynaptic induction of BDNF-mediated long-term potentiation. Science 2002; 295: 1729–1734.
Dragunow M, Beilharz E, Mason B, Lawlor P, Abraham W, Gluckman P . Brain-derived neurotrophic factor expression after long-term potentiation. Neurosci Lett 1993; 160: 232–236.
Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol 2013; 16: 301–311.
Jourdi H, Hsu YT, Zhou M, Qin Q, Bi X, Baudry M . Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J Neurosci 2009; 29: 8688–8697.
Legutko B, Li X, Skolnick P . Regulation of BDNF expression in primary neuron culture by LY392098, a novel AMPA receptor potentiator. Neuropharmacology 2001; 40: 1019–1027.
Monteggia LM, Gideons E, Kavalali ET . The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry 2013; 73: 1199–1203.
Duman RS, Li N, Liu RJ, Duric V, Aghajanian G . Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 2012; 62: 35–41.
Kavalali ET, Monteggia LM . Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry 2012; 169: 1150–1156.
Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuro-Psychoph 2011; 35: 1774–1779.
Livingstone M, Atas E, Meller A, Sonenberg N . Mechanisms governing the control of mRNA translation. Phys Biol 2010; 7: 021001.
Hoeffer CA, Klann E . mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 2010; 33: 67–75.
Chung J, Kuo CJ, Crabtree GR, Blenis J . Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 1992; 69: 1227–1236.
Heitman J, Movva N, Hall MN . Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science (New York, NY) 1991; 253: 905–909.
Carrier N, Kabbaj M . Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 2013; 70C: 27–34.
Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K et al. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci 2004; 24: 9760–9769.
Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell 2006; 22: 159–168.
Sengupta S, Peterson TR, Sabatini DM . Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 2010; 40: 310–322.
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol 2004; 14: 1296–1302.
Loix S, De Kock M, Henin P . The anti-inflammatory effects of ketamine: state of the art. Acta anaesthesiol Belg 2011; 62: 47–58.
Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM . Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 722–729.
Zunszain PA, Hepgul N, Pariante CM . Inflammation and depression. Curr Top Behav Neurosci 2013; 14: 135–151.
Hepgul N, Cattaneo A, Zunszain PA, Pariante CM . Depression pathogenesis and treatment: what can we learn from blood mRNA expression? BMC Med 2013; 11: 28.
Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology 2013; 38: 377–385.
Dale O, Somogyi AA, Li Y, Sullivan T, Shavit Y . Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg 2012; 115: 934–943.
Kawasaki T, Ogata M, Kawasaki C, Ogata J, Inoue Y, Shigematsu A . Ketamine suppresses proinflammatory cytokine production in human whole blood in vitro. Anesth Analg 1999; 89: 665–669.
Taniguchi T, Kanakura H, Takemoto Y, Kidani Y, Yamamoto K . Effects of ketamine and propofol on the ratio of interleukin-6 to interleukin-10 during endotoxemia in rats. Tohoku J Exp Med 2003; 200: 85–92.
Lankveld DP, Bull S, Van Dijk P, Fink-Gremmels J, Hellebrekers LJ . Ketamine inhibits LPS-induced tumour necrosis factor-alpha and interleukin-6 in an equine macrophage cell line. Vet Res 2005; 36: 257–262.
Li CY, Chou TC, Wong CS, Ho ST, Wu CC, Yen MH et al. Ketamine inhibits nitric oxide synthase in lipopolysaccharide-treated rat alveolar macrophages. Can J Anaesth 1997; 44: 989–995.
Welters ID, Hafer G, Menzebach A, Muhling J, Neuhauser C, Browning P et al. Ketamine inhibits transcription factors activator protein 1 and nuclear factor-kappaB, interleukin-8 production, as well as CD11b and CD16 expression: studies in human leukocytes and leukocytic cell lines. Anesth Analg 2010; 110: 934–941.
Wu Y, Li W, Zhou C, Lu F, Gao T, Liu Y et al. Ketamine inhibits lipopolysaccharide-induced astrocytes activation by suppressing TLR4/NF-kB pathway. Cell Physiol Biochem 2012; 30: 609–617.
Chang HC, Lin KH, Tai YT, Chen JT, Chen RM . Lipoteichoic acid-induced TNF-alpha and IL-6 gene expressions and oxidative stress production in macrophages are suppressed by ketamine through downregulating Toll-like receptor 2-mediated activation of ERK1/2 and NFkappaB. Shock 2010; 33: 485–492.
Hoetzel A, Schmidt R . Regulatory role of anesthetics on heme oxygenase-1. Curr Drug Targets 2010; 11: 1495–1503.
DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry 2010; 71: 1605–1611.
Thakurta RG, Das R, Bhattacharya AK, Saha D, Sen S, Singh OP et al. Rapid response with ketamine on suicidal cognition in resistant depression. Indian J Psychol Med 2012; 34: 170–175.
Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res 2008; 42: 151–157.
Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry 2009; 66: 287–292.
Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 2013; 38: 743–752.
Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM et al. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 2012; 37: 939–949.
O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 2009; 14: 511–522.
Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology, advance online publication, 3 April 2013; doi:10.1038/npp.2013.71 (e-pub ahead of print).
Beurel E, Song L, Jope R . Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Molecular psychiatry 2011; 16: 1068–1070.
Klein PS, Melton DA . A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA 1996; 93: 8455–8459.
Li X, Jope RS . Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology 2010; 35: 2143–2154.
Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA . Inhibition of glycogen-synthase kinase-3 by insulin-mediated by protein-kinase-B. Nature 1995; 378: 785–789.
Sutherland C, Leighton IA, Cohen P . Inactivation of glycogen-synthase kinase-3-beta by phosphorylation—new kinase connections in insulin and growth-factor signaling. Biochem J 1993; 296: 15–19.
Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM . The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry 2009; 14: 51–59.
Ma XC, Dang YH, Jia M, Ma R, Wang F, Wu J et al. Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One 2013; 8: e56053.
Bunney BG, Bunney WE . Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry 2012; 73: 1164–1171.
Bellet MM, Vawter MP, Bunney BG, Bunney WE, Sassone-Corsi P . Ketamine influences CLOCK:BMAL1 function leading to altered circadian gene expression. PLoS One 2011; 6: e23982.
Cao R, Li A, Cho HY, Lee B, Obrietan K . Mammalian target of rapamycin signaling modulates photic entrainment of the suprachiasmatic circadian clock. J Neurosci 2010; 30: 6302–6314.
Colwell CS, Ralph MR, Menaker M . Do NMDA receptors mediate the effects of light on circadian behavior? Brain Res 1990; 523: 117–120.
Winters WD, Hance AJ, Cadd GC, Lakin ML . Seasonal and sex influences on ketamine-induced analgesia and catalepsy in the rat—a possible role for melatonin. Neuropharmacology 1986; 25: 1095–1101.
Morgan CJA, Perry EB, Cho HS, Krystal JH, D'Souza DC . Greater vulnerability to the amnestic effects of ketamine in males. Psychopharmacology 2006; 187: 405–414.
Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW . An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 2008; 28: 631–637.
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013; 38: 729–742.
Acknowledgements
PAZ is supported by a NARSAD Young Investigator Award. CMP is funded by the Medical Research Council, UK (MR/J002739/1) and the National Institute for Health Research (NIHR) Biomedical Research Centre in Mental Health at South London and Maudsley NHS Foundation Trust and King's College London.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
PAZ has received speaker fees from Servier. CMP has received fees as a speaker or as a member of advisory board, as well as research funding, from pharmaceutical companies that commercialize or are developing antidepressants, such as Lilly, Servier and Janssen.
PowerPoint slides
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Zunszain, P., Horowitz, M., Cattaneo, A. et al. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase-3 as underlying mechanisms of its antidepressant properties. Mol Psychiatry 18, 1236–1241 (2013). https://doi.org/10.1038/mp.2013.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2013.87
Keywords
This article is cited by
-
Elucidating the Possible Role of FoxO in Depression
Neurochemical Research (2021)
-
Studying pre-treatment and ketamine-induced changes in white matter microstructure in the context of ketamine’s antidepressant effects
Translational Psychiatry (2020)
-
Ketamine relieves depression-like behaviors induced by chronic postsurgical pain in rats through anti-inflammatory, anti-oxidant effects and regulating BDNF expression
Psychopharmacology (2020)
-
The selective GSK3 inhibitor, SAR502250, displays neuroprotective activity and attenuates behavioral impairments in models of neuropsychiatric symptoms of Alzheimer’s disease in rodents
Scientific Reports (2019)
-
Protein kinase Mζ in medial prefrontal cortex mediates depressive-like behavior and antidepressant response
Molecular Psychiatry (2018)