Abstract
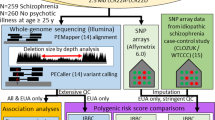
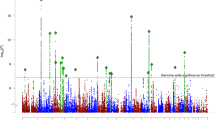
Recent molecular studies have implicated common alleles of small to moderate effect and rare alleles with larger effect sizes in the genetic architecture of schizophrenia (SCZ). It is expected that the reliable detection of risk variants with very small effect sizes can only be achieved through the recruitment of very large samples of patients and controls (that is tens of thousands), or large, potentially more homogeneous samples that have been recruited from confined geographical areas using identical diagnostic criteria. Applying the latter strategy, we performed a genome-wide association study (GWAS) of 1169 clinically well characterized and ethnically homogeneous SCZ patients from a confined area of Western Europe (464 from Germany, 705 from The Netherlands) and 3714 ethnically matched controls (1272 and 2442, respectively). In a subsequent follow-up study of our top GWAS results, we included an additional 2569 SCZ patients and 4088 controls (from Germany, The Netherlands and Denmark). Genetic variation in a region on chromosome 11 that contains the candidate genes AMBRA1, DGKZ, CHRM4 and MDK was significantly associated with SCZ in the combined sample (n=11 540; P=3.89 × 10−9, odds ratio (OR)=1.25). This finding was replicated in 23 206 independent samples of European ancestry (P=0.0029, OR=1.11). In a subsequent imaging genetics study, healthy carriers of the risk allele exhibited altered activation in the cingulate cortex during a cognitive control task. The area of interest is a critical interface between emotion regulation and cognition that is structurally and functionally abnormal in SCZ and bipolar disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sullivan PF, Kendler KS, Neale MC . Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 2003; 60: 1187–1192.
Williams HJ, Owen MJ, O’Donovan MC . New findings from genetic association studies of schizophrenia. J Hum Genet 2009; 54: 9–14.
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al. Common variants conferring risk of schizophrenia. Nature 2009; 460: 744–747.
International Schizophrenia Consortium, Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009; 460: 748–752.
Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe’er I et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 2009; 460: 753–757.
Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet 2003; 73: 34–48.
Brown AS, Derkits EJ . Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167: 261–280.
O’Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V et al. Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet 2008; 40: 1053–1055.
First MB, Spitzer RL, Gibbon M, Williams JBW . Structured Clinical Interview for DSM-IV Axis I Disorders. New York State Psychiatric Institute, Biometrics Research: New York, 1994.
Spitzer RL, Endicott J, Robins E . Research diagnostic criteria: rationale and reliability. Arch Gen Psychiatry 1978; 35: 773–782.
McGuffin P, Farmer A, Harvey I . A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry 1991; 48: 764–770.
Krawczak M, Nikolaus S, von Eberstein H, Croucher PJ, El Mokhtari NE, Schreiber S . PopGen: population-based recruitment of patients and controls for the analysis of complex genotype-phenotype relationships. Community Genet 2006; 9: 55–61.
Wichmann HE, Gieger C, Illig T . KORA-gen – Resource for population genetics, controls and a broad spectrum of disease phenotypes. KORA- gen – Ressource für Bevölkerungsgenetik, Kontrolle und ein breites Spektrum an Krankheitsphänotypen. Gesundheitswesen 2005; 67: S26–S30.
Schmermund A, Möhlenkamp S, Stang A, Grönemeyer D, Seibel R, Hirche H et al. Assessment of clinically silent atherosclerotic disease and established and novel risk factors for predicting myocardial infarction and cardiac death in healthy middle-aged subjects: rationale and design of the Heinz Nixdorf Recall Study. Am Heart J 2002; 144: 212–218.
Andreasen NC, Flaum M, Arndt S . The comprehensive assessment of symptoms and history (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49: 615–623.
Hofman A, Breteler MM, van Duijn CM, Krestin GP, Pols HA, Stricker BH et al. The Rotterdam Study: objectives and design update. Eur J Epidemiol 2007; 22: 819–829.
Hofman A, Grobbee DE, de Jong PT, van den Ouweland FA . Determinants of disease and disability in the elderly: the Rotterdam Elderly Study. Eur J Epidemiol 1991; 7: 403–422.
Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C et al. Neural mechanisms of a genome-wide supported psychosis variant. Science 2009; 324: 605.
Miller SA, Dykes DD, Polesky HF . A simple salting out precedure for extracting DNA from Human nucleated cells. Nucleic Acids Res 1988; 16: 1215.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
Erk S, Meyer-Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P et al. Altered neural function in carriers of a bipolar disorder variant with genome-wide support. Arch Gen Psychiatry 2010; 67: 803–811.
Callicott JH, Ramsey NF, Tallent K, Bertolino A, Knable MB, Coppola R et al. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology 1998; 18: 186–196.
Walter H, Schnell K, Erk S, Arnold C, Kirsch P, Esslinger C et al. Effects of a genome-wide supported psychosis risk variant on neural activation during a theory-of-mind task. Mol Psychiatry 2011; 16: 462–470.
Meyer-Lindenberg A, Buckholtz JW, Kolachana B, R Hariri A, Pezawas L, Blasi G et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 2006; 103: 6269–6274.
Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR . The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage 2002; 17: 317–323.
Kirsch P, Schienle A, Stark R, Sammer G, Blecker C, Walter B et al. Anticipation of reward in a nonaversive differential conditioning paradigm and the brain reward system: an eventrelated fMRI study. Neuroimage 2003; 20: 1086–1095.
The International HapMap Consortium. A haplotype map of the human genome. Nature 2005; 437: 1299–1320.
Behrends C, Sowa ME, Gygi SP, Harper JW . Network organization of the human autophagy system. Nature 2010; 466: 68–76.
Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R et al. Ambra1 regulates autophagy and development of the nervous system. Nature 2007; 447: 1121–1125.
Cecconi F, Di Bartolomeo S, Nardacci R, Fuoco C, Corazzari M, Giunta L et al. A novel role for autophagy in neurodevelopment. Autophagy 2007; 3: 506–508.
Scarr E . Muscarinic receptors in psychiatric disorders - can we mimic ‘Health’? Neurosignals 2009; 17: 298–310.
Raedler TJ, Tandon R . Cholinergic mechanisms in schizophrenia: current concepts. Curr Psychosis Therapeutics Rep 2006; 4: 20–26.
Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC et al. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci USA 2008; 105: 10978–10983.
Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E et al. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol Psychiatry 2009; 14: 252–260.
Baum AE, Akula N, Cabanero M, Cardona I, Corona W, Klemens B et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry 2008; 13: 197–207.
Hozumi Y, Watanabe M, Otani K, Goto K . Diacylglycerol kinase beta promotes dendritic outgrowth and spine maturation in developing hippocompal neurons. BMC Neurosci 2009; 10: 99.
Nakamura E, Kadomatsu K, Yuasa S, Muramatsu H, Mamiya T, Nabeshima T et al. Disruption of the midkine gene (Mdk) resulted in altered expression of a calcium binding protein in the hippocampus of infant mice and their abnormal behaviour. Genes Cells 1998; 3: 811–822.
Yasuhara O, Muramatsu H, Kim SU, Muramatsu T, Maruta H, McGeer PL . Midkine, a novel neurpotropic factor, is present in senile plaques of Alzheimer disease. Biochem Biophys Res Commun 1993; 192: 246–251.
Ohgake S, Shimizu E, Hashinoto K, Okamura N, Koike K, Koizumi H et al. Dopaminergic hypofunctions and preulse inhibition deficits in mice lacking midkine. Prog Neuropsychopharmacol Biol Psychiatry 2009; 33: 541–546.
Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL . Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology (Berl) 2008; 199: 331–388.
Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O’Donnell CJ, de Bakker PI . SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 2008; 24: 2938–2939.
Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR . False positives in imaging genetics. NeuroImage 2008; 40: 655–661.
Koo MS, Levitt JJ, Salisbury DF, Nakamura M, Shenton ME, McCarley RW . A cross-sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first-episode schizophrenia and first-episode affective psychosis. Arch Gen Psychiatry 2008; 65: 746–760.
Rasetti R, Mattay VS, Wiedholz LM, Kolachana BS, Hariri AR, Callicott JH et al. Evidence that altered amygdala activity in schizophrenia is related to clinical state and not genetic risk. Am J Psychiatry 2009; 166: 216–225.
Acknowledgements
We are grateful to all of the patients who contributed to this study. We also thank the probands from the community-based cohorts of PopGen, KORA, the Heinz Nixdorf Recall (HNR) study, the Rotterdam Elderly Study, as well as the Munich controls. This study was supported by the German Federal Ministry of Education and Research (BMBF), within the context of the National Genome Research Network plus (NGFNplus) and the MooDS-Net (grant 01GS08144 to SC and MMN, grant 01GS08147 to MR). MMN also received support from the Alfried Krupp von Bohlen und Halbach-Stiftung. Further funding came from the European Union Seventh Framework Programme (FP7/2007–2011) under grant agreement no. 242257 (ADAMS). The Heinz Nixdorf Recall cohort was established with the support of the Heinz Nixdorf Foundation (Dr G Schmidt, Chairman). This study was also supported by The Polish Ministry of Science and Higher Education (grant N N402 244035 to PMC), The Danish Council for Strategic Research (grant no. 2101–07-0059), H Lundbeck A/S, the Faculty of Health Sciences, Aarhus University and the Stanley Medical Research Institute. Genotyping of the Dutch samples from Utrecht was sponsored by NIMH funding, R01 MH078075.
Author information
Authors and Affiliations
Consortia
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
René S Kahn1, Don H Linszen2, Jim van Os3, Durk Wiersma4, Richard Bruggeman4, Wiepke Cahn1, Lieuwe de Haan2, Lydia Krabbendam3 and Inez Myin-Germeys3 1Department of Psychiatry, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, Utrecht, The Netherlands;2Academic Medical Centre University of Amsterdam, Department of Psychiatry, Amsterdam, The Netherlands;3Maastricht University Medical Centre, South Limburg Mental Health Research and Teaching Network, Maastricht, The Netherlands and4University Medical Center Groningen, Department of Psychiatry, University of Groningen, Groningen, The Netherlands.
Hreinn Stefansson1, Stacy Steinberg1, Omar Gustafsson1, Engilbert Sigurdsson2, Hannes Petursson2, Augustine Kong1, Kari Stefansson1, Olli PH Pietiläinen3,4, Annamari Tuulio-Henriksson5, Tiina Paunio6, Jouko Lonnqvist5, Jaana Suvisaari5, Leena Peltonen3,4, Mirella Ruggeri7, Sarah Tosato7, Muriel Walshe8, Robin Murray8, David A Collier8, David St Clair9, Thomas Hansen10, Andres Ingason10, Klaus D Jakobsen10, Linh Duong10, Thomas Werge10, Ingrid Melle11, Ole A Andreassen12, Srdjan Djurovic12, István Bitter13, János M Réthelyi13, Lilia Abramova14, Vasily Kaleda14, Vera Golimbet14, Erik G Jönsson15, Lars Terenius15, Ingrid Agartz15, Ruud van Winkel16,17, Gunter Kenis16, Marc De Hert17, Jan Veldink18, Carsten Wiuf19 and Michael Didriksen20 1deCODE genetics, Sturlugata 8, IS-101 Reykjavik, Iceland;2Department of Psychiatry, National University Hospital, Hringbraut, IS-101 Reykjavik, Iceland;3Institute for Molecular Medicine Finland FIMM and Department of Medical Genetics, University of Helsinki, FI-00014 Helsinki, Finland;4Wellcome Trust Sanger Institute, Cambridge CB10 1SA, UK;5Department of Mental Health and Alcohol Research, National Public Health Institute, Mannerheimintie 166, FI-00300 Helsinki, Finland;6Health Genomics Unit, National Institute for Health and Welfare THL, FI-00270 Helsinki, Finland;7Section of Psychiatry and Clinical Psychology, University of Verona, 37134 Verona, Italy;8Institute of Psychiatry, King's College, London SE5 8AF, UK;9Department of Mental Health, University of Aberdeen, Royal Cornhill Hospital, Aberdeen AB25 2ZD, UK;10Institute of Biological Psychiatry, Mental Health Centre Sct. Hans Copenhagen University Hospital, DK-4000 Roskilde, Denmark;11Department of Medical Genetics, Ulleval University Hospital, Institute of Psychiatry, University of Oslo, Kirkeveien 166, N-0407 Oslo, Norway;12Department of Psychiatry, Ullevål University Hospital and Institute of Psychiatry, University of Oslo, Kirkeveien 166, N-0407 Oslo, Norway;13Semmelweis University, Department of Psychiatry and Psychotherapy, Budapest, Hungary;14Mental Health Research Center, Russian Academy of Medical Sciences, Zagorodnoe sh. 2/2, 117152 Moscow, Russia;15Department of Clinical Neuroscience, HUBIN project, Karolinska Institutet and Hospital, R5:00, SE-171 76 Stockholm, Sweden;16Department of Psychiatry and Psychology, School of Mental Health and Neuroscience, European Graduate School of Neuroscience (EURON), South Limburg Mental Health Research and Teaching Network (SEARCH), Maastricht University Medical Centre, 6200 MD Maastricht, The Netherlands;17University Psychiatric Centre Catholic University Leuven, Leuvensesteenweg 517, B-3070 Kortenberg, Belgium;18Rudolf Magnus Institute of Neuroscience, Department of Neurology, UMC Utrecht, 3584 CG Utrecht, The Netherlands;19Bioinformatics Research Centre, Aarhus University, DK-8000 Aarhus C, Denmark and20Discovery Biology Research, H Lundbeck A/S, Ottiliavej 9, DK-2500 Valby, Denmark.
Supplementary Information accompanies the paper on the Molecular Psychiatry website
PowerPoint slides
Rights and permissions
About this article
Cite this article
Rietschel, M., Mattheisen, M., Degenhardt, F. et al. Association between genetic variation in a region on chromosome 11 and schizophrenia in large samples from Europe. Mol Psychiatry 17, 906–917 (2012). https://doi.org/10.1038/mp.2011.80
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2011.80
Keywords
This article is cited by
-
Neurodevelopmental Disorders: Functional Role of Ambra1 in Autism and Schizophrenia
Molecular Neurobiology (2019)
-
Expression analysis and genotyping of DGKZ: a GWAS-derived risk gene for schizophrenia
Molecular Biology Reports (2019)
-
Sexual dimorphism of AMBRA1-related autistic features in human and mouse
Translational Psychiatry (2017)
-
Evaluation of European Schizophrenia GWAS Loci in Asian Populations via Comprehensive Meta-Analyses
Molecular Neurobiology (2017)
-
A human-specific AS3MT isoform and BORCS7 are molecular risk factors in the 10q24.32 schizophrenia-associated locus
Nature Medicine (2016)