Abstract
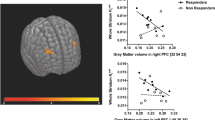
Schizophrenia is characterized by altered prefrontal activity and elevated striatal dopaminergic function. To investigate the relationship between these abnormalities in the prodromal phase of the illness, we combined functional Magnetic Resonance Imaging and 18F-Dopa Positron Emission Tomography. When performing a verbal fluency task, subjects with an At-Risk Mental State showed greater activation in the inferior frontal cortex than controls. Striatal dopamine function was greater in the At-Risk group than in controls. Within the At-Risk group, but not the control group, there was a direct correlation between the degree of left inferior frontal activation and the level of striatal dopamine function. Altered prefrontal activation in subjects with an At-Risk Mental State for psychosis is related to elevated striatal dopamine function. These changes reflect an increased vulnerability to psychosis and predate the first episode of frank psychosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Laruelle M . Schizophrenia is associated with increased synaptic dopamine in associative rather than limbic regions of the striatum: implications for the mechanisms of actions of antipsychotic drugs. Schizophr Res 2006; 81: 16.
Howes OD, Montgomery AJ, Asselin M, Murray R, Grasby P, McGuire P . Molecular imaging studies of the striatal dopaminergic system in psychosis and predictions for the prodromal phase of psychosis. Br J Psychiatry 2007; S51: s13–s18.
Weinberger DR, Berman KF . Prefrontal function in schizophrenia: confounds and controversies. Philos Trans R Soc Lond B Biol Sci 1996; 351: 1495–1503.
Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry 2001; 50: 825–844.
Curtis VA, Bullmore ET, Brammer MJ, Wright IC, Williams SC, Morris RG et al. Attenuated frontal activation during a verbal fluency task in patients with schizophrenia. Am J Psychiatry 1998; 155: 1056–1063.
Fu CH, Morgan K, Suckling J, Williams SC, Andrew C, Vythelingum GN et al. A functional magnetic resonance imaging study of overt letter verbal fluency using a clustered acquisition sequence: greater anterior cingulate activation with increased task demand. Neuroimage 2002; 17: 871–879.
Reichenberg A, Harvey PD . Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychol Bull 2007; 133: 833–858.
Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell'Olio M et al. Mapping the onset of psychosis: the Comprehensive Assessment of At-Risk Mental States. Aust N Z J Psychiatry 2005; 39: 964–971.
Cannon TD, Cornblatt B, McGorry P . The empirical status of the ultra high-risk (prodromal) research paradigm. Schizophr Bull 2007; 33: 661–664.
Phillips LJ, McGorry PD, Yung AR, McGlashan TH, Cornblatt B, Klosterkotter J . Prepsychotic phase of schizophrenia and related disorders: recent progress and future opportunities. Br J Psychiatry Suppl 2005; 48: s33–s44.
Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L et al. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry 2006; 59: 863–871.
Pukrop R, Schultze-Lutter F, Ruhrmann S, Brockhaus-Dumke A, Tendolkar I, Bechdolf A et al. Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J Clin Exp Neuropsychol 2006; 28: 1388–1407.
Simon AE, Cattapan-Ludewig K, Zmilacher S, Arbach D, Gruber K, Dvorsky DN et al. Cognitive functioning in the schizophrenia prodrome. Schizophr Bull 2007; 33: 761–771.
Fusar-Poli P, Perez J, Broome MR, Borgwardt S, Placentino A, Caverzasi E et al. Neurofunctional correlates of liability to psychosis: a systematic review and meta-analysis. Neur Biob Rev 2007; 31: 465–484.
Howes O, Montgomery A, Asselin M, Valli I, Tabraham P, Johns L et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of General Psychiatry 2009; 66: 13–20.
Tepper JM, Bolam JP . Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol 2004; 14: 685–692.
Holt DJ, Graybiel AM, Saper CB . Neurochemical architecture of the human striatum. J Comp Neurol 1997; 384: 1–25.
Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM . Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 2004; 27: 468–474.
Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci 2002; 5: 267–271.
Alves Fda S, Figee M, Vamelsvoort T, Veltman D, Haan L . The revised dopamine hypothesis of schizophrenia: evidence from pharmacological MRI studies with atypical antipsychotic medication. Psychopharmacol Bull 2008; 41: 121–132.
Vita A, De Peri L . The effects of antipsychotic treatment on cerebral structure and function in schizophrenia. Int Rev Psychiatry 2007; 19: 429–436.
Morey RA, Inan S, Mitchell TV, Perkins DO, Lieberman JA, Belger A . Imaging frontostriatal function in ultra-high-risk, early, and chronic schizophrenia during executive processing. Arch Gen Psychiatry 2005; 62: 254–262.
Broome M, Woolley J, Tabraham P, Johns L, Bramon E, Murray G et al. What causes the onset of psychosis? Schizophr Res 2005; 79: 23–34.
Coren S . Measurement of handedness via self-report: the relationship between brief and extended inventories. Percept Mot Skills 1993; 76 (3 Pt 1): 1035–1042.
Kay SR . Positive-negative symptom assessment in schizophrenia: psychometric issues and scale comparison. Psychiatr Q 1990; 61: 163–178.
Barkus EJ, Stirling J, Hopkins RS, Lewis S . Cannabis-induced psychosis-like experiences are associated with high schizotypy. Psychopathology 2006; 39: 175–178.
Nelson HE, Willison JR . National Adult Reading Test (NART): Test Manual 2nd ed. NFER-Nelson Windsor: England, 1991.
Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS . A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp 2006; 27: 799–810.
Maldjian J, Laurienti P, Kraft R, Burdette J . An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 2003; 19: 1233–1239.
Spinks TJ, Jones T, Bloomfield PM, Bailey DL, Miller M, Hogg D et al. Physical characteristics of the ECAT EXACT3D positron tomograph. Phys Med Biol 2000; 45: 2601–2618.
Ishikawa T, Dhawan V, Chaly T, Robeson W, Belakhlef A, Mandel F et al. Fluorodopa positron emission tomography with an inhibitor of catechol-O-methyltransferase: effect of the plasma 3-O-methyldopa fraction on data analysis. J Cereb Blood Flow Metab 1996; 16: 854–863.
Sawle GV, Burn DJ, Morrish PK, Lammertsma AA, Snow BJ, Luthra S et al. The effect of entacapone (OR-611) on brain -6-L-fluorodopa metabolism: implications for levodopa therapy of Parkinson's disease. Neurology 1994; 44: 1292–1297.
Wahl L, Chirakal R, Firnau G, Garnett ES, Nahmias C . The distribution and kinetics of [18F]6-fluoro-3-O-methyl-L-dopa in the human brain. J Cereb Blood Flow Metab 1994; 14: 664–670.
Turkheimer FE, Brett M, Visvikis D, Cunningham VJ . Multiresolution analysis of emission tomography images in the wavelet domain. J Cereb Blood Flow Metab 1999)); 19: 1189–1208.
Studholme C, Hill DL, Hawkes DJ . Automated 3-D registration of MR and CT images of the head. Med Image Anal 1996; 1: 163–175.
Montgomery AJ, Thielemans K, Mehta MA, Turkheimer F, Mustafovic S, Grasby PM . Correction of head movement on PET studies: comparison of methods. J Nucl Med 2006; 47: 1936–1944.
Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp 2003; 19: 224–247.
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21: 1034–1057.
McGowan S, Lawrence AD, Sales T, Quested D, Grasby P . Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic fluorodopa study. Arch Gen Psychiatry 2004; 61: 134–142.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y et al. Imaging human mesolimbic dopamine transmission with positron emission tomography.Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 2003; 23: 285–300.
Patlak CS, Blasberg RG, Fenstermacher JD . Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983; 3: 1–7.
Moore RY, Whone AL, McGowan S, Brooks DJ . Monoamine neuron innervation of the normal human brain: an 18F-DOPA PET study. Brain Res 2003; 982: 137–145.
Fu CH, Suckling J, Williams SC, Andrew CM, Vythelingum GN, McGuire PK . Effects of psychotic state and task demand on prefrontal function in schizophrenia: an fMRI study of overt verbal fluency. Am J Psychiatry 2005; 162: 485–494.
Price CJ, Friston KJ . Scanning patients with tasks they can perform. Hum Brain Mapp 1999; 8: 102–108.
Tan HY, Callicott JH, Weinberger DR . Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex 2007; 17 Suppl 1: i171–i181.
Fusar-Poli P, Broome M, Matthiasson P, Woolley J, Mechelli A, Johns L et al. Prefrontal response during executive functioning at presentation directly related to twelve months clinical outcome in people at ultra high risk of psychosis. Schizophrenia Bulletin; 7 August 2009 [e-pub ahead of print].
Borgwardt SJ, Riecher-Rossler A, Dazzan P, Chitnis X, Aston J, Drewe M et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry 2007; 61: 1148–1156.
Meisenzahl EM, Koutsouleris N, Gaser C, Bottlender R, Schmitt GJ, McGuire P et al. Structural brain alterations in subjects at high-risk of psychosis: a voxel-based morphometric study. Schizophr Res 2008; 102: 150–162.
Garcia-Marti G, Aguilar EJ, Lull JJ, Marti-Bonmati L, Escarti MJ, Manjon JV et al. Schizophrenia with auditory hallucinations: a voxel-based morphometry study. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 72–80.
Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R et al. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry 2005; 58: 457–467.
Borgwardt SJ, McGuire PK, Aston J, Berger G, Dazzan P, Gschwandtner U et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry Suppl 2007; 51: s69–s75.
Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 2003; 361: 281–288.
Sun D, Phillips L, Velakoulis D, Yung A, McGorry P, Wood S et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res 2009; 108: 85–92.
Laruelle M, Abi-Dargham A . Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol 1999; 13: 358–371.
Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R . Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 1999; 46: 56–72.
Alexander GE, DeLong MR, Strick PL . Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9: 357–381.
Fusar-Poli P, Allen P, McGuire P . Neuroimaging studies of the early stages of psychosis: a critical review. Eur Psychiatry 2008; 23: 237–244.
Prata D, Mechelli A, Fu C, Picchioni M, Kane F, Kalidindi S et al. Opposite effects of COMT Val158Met on cortical function inof healthy subjects and patients with schizophrenia. Biol Psychiatry 2008 (in press).
Nakano K, Kayahara T, Tsutsumi T, Ushiro H . Neural circuits and functional organization of the striatum. J Neurol 2000; 247 Suppl 5: 6–15.
Guillin O, Abi-Dargham A, Laruelle M . Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol 2007; 78: 1–39.
Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML . Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol 2001; 41: 237–260.
Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 2006; 49: 603–615.
Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry 2008; 65: 28–37.
Howes O, Kapur S . The Dopamine Hypothesis of Schizophrenia: Version III -The Final Common Pathway. Schizophr Bull 2009; 35: 549–562.
Meisenzahl EM, Schmitt GJ, Scheuerecker J, Moller HJ . The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry 2007; 19: 337–345.
Acknowledgements
We acknowledge the contribution of the volunteers who participated in the study, the radiographers who assisted with the imaging, and clinical professionals at OASIS. The Medical Research Council, UK and Psychiatry Research Trust provided funding for this study. PFP takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have agreed to its submission in this form.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fusar-Poli, P., Howes, O., Allen, P. et al. Abnormal prefrontal activation directly related to pre-synaptic striatal dopamine dysfunction in people at clinical high risk for psychosis. Mol Psychiatry 16, 67–75 (2011). https://doi.org/10.1038/mp.2009.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2009.108
Keywords
This article is cited by
-
Reduced Number Density of Oligodendrocytes and Oligodendrocyte Clusters in the Head of the Caudate Nucleus in Schizophrenia
Neuroscience and Behavioral Physiology (2023)
-
Microglia sequelae: brain signature of innate immunity in schizophrenia
Translational Psychiatry (2022)
-
The relationship between glutamate, dopamine, and cortical gray matter: A simultaneous PET-MR study
Molecular Psychiatry (2022)
-
Integrating trauma, self-disturbances, cognitive biases, and personality into a model for the risk of psychosis: a longitudinal study in a non-clinical sample
European Archives of Psychiatry and Clinical Neuroscience (2022)
-
The relationship between grey matter volume and striatal dopamine function in psychosis: a multimodal 18F-DOPA PET and voxel-based morphometry study
Molecular Psychiatry (2021)