Abstract
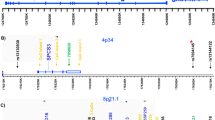
Several independent linkage studies have identified chromosome 4p15–p16 as a putative region of susceptibility for bipolar disorder (BP), schizophrenia (SCZ) and related phenotypes. Previously, we identified two subregions (B and D) of the 4p15–p16 region that are shared by three of four 4p-linked families examined. Here, we describe a large-scale association analysis of regions B and D (3.8 and 4.5 Mb, respectively). We selected 408 haplotype-tagging single nucleotide polymorphisms (SNPs) on a block-by-block basis from the International HapMap project and tested them in 368 BP, 386 SCZ and 458 control individuals. Nominal significance thresholds were determined using principal component analysis as implemented in the program SNPSpD. In region B, overlapping SNPs and haplotypes met the region-wide threshold (P⩽0.0005) at the global and individual haplotype test level and clustered in two regions. In region D, no individual SNPs were nominally significant, but multiple global and individual haplotypes were associated with BP and/or SCZ (region-wide threshold, P⩽0.0003). These overlapping haplotypes fell into two regions. Within each of these four clusters, at least one globally significant haplotype withstood permutation testing (Pgp⩽0.05). Five predicted genes were found within these associated regions, while Known/RefSeq genes, including KIAA0746 and PPARGC1A, mapped nearby. There were also nine other clusters within regions B and D with nominally significant haplotypes, but only at the individual haplotype level. KIAA0746, PPARGC1A, GPR125, CCKAR and DKFZp761B107 overlapped with these regions. This study has identified significant associations between BP and SCZ within the chromosome 4p linkage region, resulting in candidate regions worthy of further investigation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lopez AD, Murray CC . The global burden of disease, 1990–2020. Nat Med 1998; 4: 1241–1243.
Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 2002; 71: 877–892.
Thomson PA, Christoforou A, Morris SW, Adie E, Pickard BS, Porteous DJ et al. Association of neuregulin 1 with schizophrenia and bipolar disorder in a second cohort from the Scottish population. Mol Psychiatry 2007; 12: 94–104.
Hennah W, Thomson P, Peltonen L, Porteous D . Genes and schizophrenia: beyond schizophrenia: the role of DISC1 in major mental illness. Schizophr Bull 2006; 32: 409–416.
Blackwood DH, He L, Morris SW, McLean A, Whitton C, Thomson M et al. A locus for bipolar affective disorder on chromosome 4p. Nat Genet 1996; 12: 427–430.
Visscher PM, Haley CS, Heath SC, Muir WJ, Blackwood DH . Detecting QTLs for uni- and bipolar disorder using a variance component method. Psychiatr Genet 1999; 9: 75–84.
Le Hellard S, Lee AJ, Underwood S, Thomson PA, Morris SW, Torrance HS et al. Haplotype analysis and a novel allele-sharing method refines a chromosome 4p locus linked to bipolar affective disorder. Biol Psychiatry 2007; 61: 797–805.
Detera-Wadleigh SD, Badner JA, Berrettini WH, Yoshikawa T, Goldin LR, Turner G et al. A high-density genome scan detects evidence for a bipolar-disorder susceptibility locus on 13q32 and other potential loci on 1q32 and 18p11.2. Proc Natl Acad Sci USA 1999; 96: 5604–5609.
Als TD, Dahl HA, Flint TJ, Wang AG, Vang M, Mors O et al. Possible evidence for a common risk locus for bipolar affective disorder and schizophrenia on chromosome 4p16 in patients from the Faroe Islands. Mol Psychiatry 2004; 9: 93–98.
Itokawa M, Kasuga T, Yoshikawa T, Matsushita M . Identification of a male schizophrenic patient carrying a de novo balanced translocation, t(4; 13)(p16.1; q21.31). Psychiatry Clin Neurosci 2004; 58: 333–337.
Asherson P, Mant R, Williams N, Cardno A, Jones L, Murphy K et al. A study of chromosome 4p markers and dopamine D5 receptor gene in schizophrenia and bipolar disorder. Mol Psychiatry 1998; 3: 310–320.
Underwood SL, Christoforou A, Thomson PA, Wray NR, Tenesa A, Whittaker J et al. Association analysis of the chromosome 4p-located G protein-coupled receptor 78 (GPR78) gene in bipolar affective disorder and schizophrenia. Mol Psychiatry 2006; 11: 384–394.
Edwards AO, Ritter III R, Abel KJ, Manning A, Panhuysen C, Farrer LA . Complement factor H polymorphism and age-related macular degeneration. Science 2005; 308: 421–424.
Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P et al. Complement factor H variant increases the risk of age-related macular degeneration. Science 2005; 308: 419–421.
Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005; 308: 385–389.
Rivera A, Fisher SA, Fritsche LG, Keilhauer CN, Lichtner P, Meitinger T et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet 2005; 14: 3227–3236.
Hennah W, Varilo T, Kestila M, Paunio T, Arajarvi R, Haukka J et al. Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet 2003; 12: 3151–3159.
Thomson PA, Wray NR, Millar JK, Evans KL, Hellard SL, Condie A et al. Association between the TRAX/DISC locus and both bipolar disorder and schizophrenia in the Scottish population. Mol Psychiatry 2005; 10: 657–668, 616.
Consortium TIH . The international HapMap project. Nature 2003; 18: 789–796.
Endicott J, Spitzer RL . A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry 1978; 35: 837–844.
Association AP . Diagnostic and Statistical Manual of Mental Disorders (4th edn) (DSM–IV). APA: Washington, DC, 1994.
Office of the Chief Statistician SE. Analysis of Ethnicity in the 2001 Census – Summary Report. Scottish Executive: Edinburgh, 2004.
Barrett JC, Fry B, Maller J, Daly MJ . Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265.
Hedrick PW . Gametic disequilibrium measures: proceed with caution. Genetics 1987; 117: 331–341.
Ke X, Durrant C, Morris AP, Hunt S, Bentley DR, Deloukas P et al. Efficiency and consistency of haplotype tagging of dense SNP maps in multiple samples. Hum Mol Genet 2004; 13: 2557–2565.
Nyholt DR . A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 2004; 74: 765–769.
Cheverud JM . A simple correction for multiple comparisons in interval mapping genome scans. Heredity 2001; 87: 52–58.
Li J, Ji L . Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 2005; 95: 221–227.
Sidak Z . Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc 1967; 62: 626–633.
Dudbridge F . Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol 2003; 25: 115–121.
Churchill GA, Doerge RW . Empirical threshold values for quantitative trait mapping. Genetics 1994; 138: 963–971.
Holm S . A simple sequentially rejective multiple test procedure. Scand J Statist 1979; 6: 65–70.
Koeleman BP, Dudbridge F, Cordell HJ, Todd JA . Adaptation of the extended transmission/disequilibrium test to distinguish disease associations of multiple loci: the Conditional Extended Transmission/Disequilibrium Test. Ann Hum Genet 2000; 64: 207–213.
Fallin MD, Lasseter VK, Avramopoulos D, Nicodemus KK, Wolyniec PS, McGrath JA et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case–parent trios. Am J Hum Genet 2005; 77: 918–936.
Greenwood TA, Schork NJ, Eskin E, Kelsoe JR . Identification of additional variants within the human dopamine-transporter gene provides further evidence for an association with bipolar disorder in two independent samples. Mol Psychiatry 2006; 11: 125–133, 115.
Thierry-Mieg D, Thierry-Mieg J, Potdevin M, Sienkiewicz M . AceView: identification and functional annotation of cDNA-supported genes in higher organisms. unpublished. The AceView genes: http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly.
Fallin D, Cohen A, Essioux L, Chumakov I, Blumenfeld M, Cohen D et al. Genetic analysis of case/control data using estimated haplotype frequencies: application to APOE locus variation and Alzheimer's disease. Genome Res 2001; 11: 143–151.
Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM et al. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proc Natl Acad Sci USA 2006; 103: 1810–1815.
Fearnhead NS, Wilding JL, Winney B, Tonks S, Bartlett S, Bicknell DC et al. Multiple rare variants in different genes account for multifactorial inherited susceptibility to colorectal adenomas. Proc Natl Acad Sci USA 2004; 101: 15992–15997.
Emison ES, McCallion AS, Kashuk CS, Bush RT, Grice E, Lin S et al. A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature 2005; 434: 857–863.
Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M et al. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet 2006; 38: 644–651.
Maller J, George S, Purcell S, Fagerness J, Altshuler D, Daly MJ et al. Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet 2006; 38: 1055–1059.
Kleinjan DA, van Heyningen V . Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet 2005; 76: 8–32.
Redden DT, Allison DB . The Effect of Assortative Mating upon Genetic Association Studies: Spurious Associations and Population Substructure in the Absence of Admixture. Behav Genet 2006; 36: 678–686.
Turakulov R, Easteal S . Number of SNPS loci needed to detect population structure. Hum Hered 2003; 55: 37–45.
Falush D, Stephens M, Pritchard JK . Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 2003; 164: 1567–1587.
Johnson GC, Esposito L, Barratt BJ, Smith AN, Heward J, Di Genova G et al. Haplotype tagging for the identification of common disease genes. Nat Genet 2001; 29: 233–237.
Daly MJ, Rioux JD, Schaffner SF, Hudson TJ, Lander ES . High-resolution haplotype structure in the human genome. Nat Genet 2001; 29: 229–232.
Ding K, Kullo IJ . Methods for the selection of tagging SNPs: a comparison of tagging efficiency and performance. Eur J Hum Genet 2007; 15: 228–236.
Goldstein DB, Ahmadi KR, Weale ME, Wood NW . Genome scans and candidate gene approaches in the study of common diseases and variable drug responses. Trends Genet 2003; 19: 615–622.
Website. Haploview Documentation: http://www.broad.mit.edu/mpg/haploview/using.php#lddisplay. 2006.
Goldstein DB . Pharmacogenetics in the laboratory and the clinic. N Engl J Med 2003; 348: 553–556.
Pe'er I, Chretien YR, de Bakker PI, Barrett JC, Daly MJ, Altshuler DM . Biases and reconciliation in estimates of linkage disequilibrium in the human genome. Am J Hum Genet 2006; 78: 588–603.
Akey J, Jin L, Xiong M . Haplotypes vs single marker linkage disequilibrium tests: what do we gain? Eur J Hum Genet 2001; 9: 291–300.
Wray NR . Allele frequencies and the r2 measure of linkage disequilibrium: impact on design and interpretation of association studies. Twin Res Hum Genet 2005; 8: 87–94.
Schaid DJ . Genetic epidemiology and haplotypes. Genet Epidemiol 2004; 27: 317–320.
Sullivan PF, Eaves LJ, Kendler KS, Neale MC . Genetic case-control association studies in neuropsychiatry. Arch Gen Psychiatry 2001; 58: 1015–1024.
Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N . Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst 2004; 96: 434–442.
Craddock N, O'Donovan MC, Owen MJ . Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull 2006; 32: 9–16.
Hamshere ML, Bennett P, Williams N, Segurado R, Cardno A, Norton N et al. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p13. Arch Gen Psychiatry 2005; 62: 1081–1088.
Van Den Bogaert A, Del-Favero J, Van Broeckhoven C . Major affective disorders and schizophrenia: a common molecular signature? Hum Mutat 2006; 27: 833–853.
Berrettini W . Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet C Semin Med Genet 2003; 123: 59–64.
Maier W, Lichtermann D, Franke P, Heun R, Falkai P, Rietschel M . The dichotomy of schizophrenia and affective disorders in extended pedigrees. Schizophr Res 2002; 57: 259–266.
Potash JB, Willour VL, Chiu YF, Simpson SG, MacKinnon DF, Pearlson GD et al. The familial aggregation of psychotic symptoms in bipolar disorder pedigrees. Am J Psychiatry 2001; 158: 1258–1264.
Holden C . Sex and the suffering brain. Science 2005; 308: 1574.
Burt VK, Rasgon N . Special considerations in treating bipolar disorder in women. Bipolar Disord 2004; 6: 2–13.
Barnes C, Mitchell P . Considerations in the management of bipolar disorder in women. Aust N Z J Psychiatry 2005; 39: 662–673.
Kennedy N, Boydell J, Kalidindi S, Fearon P, Jones PB, van Os J et al. Gender differences in incidence and age at onset of mania and bipolar disorder over a 35-year period in Camberwell, England. Am J Psychiatry 2005; 162: 257–262.
Riecher-Rossler A, Hafner H . Gender aspects in schizophrenia: bridging the border between social and biological psychiatry. Acta Psychiatr Scand Suppl 2000; 407: 58–62.
Hafner H . Gender differences in schizophrenia. Psychoneuroendocrinology 2003; 28 (Suppl 2): 17–54.
Viguera AC, Tondo L, Baldessarini RJ . Sex differences in response to lithium treatment. Am J Psychiatry 2000; 157: 1509–1511.
Hori H, Ohmori O, Matsumoto C, Shinkai T, Nakamura J . NAD(P)H: quinone oxidoreductase (NQO1) gene polymorphism and schizophrenia. Psychiatry Res 2003; 118: 235–239.
Zubenko GS, Hughes III HB, Stiffler JS, Brechbiel A, Zubenko WN, Maher BS et al. Sequence variations in CREB1 cosegregate with depressive disorders in women. Mol Psychiatry 2003; 8: 611–618.
Acknowledgements
This work was supported from grants from the Chief Scientists Office, Scottish Executive; the Health Foundation, London; the Medical Research Council, UK and the Wellcome Trust. We thank the patients, their families and volunteers for their participation in this study. We thank Maura Walker, Margaret van Beck, Sally Roe and Susan Jackson for the collation of patient data. We thank Kirsty Millar, Helen Torrance, Susan Anderson, Alison Condie, John Beekman, Pat Malloy, Alan MacLean, Rosalind Launchbury, Sebastienne Buchanan and the Wellcome Trust CRF Genetics Core for their help in the preparation of the samples. We thank Illumina, San Diego, for genotyping our samples. We thank Richard Adams for bioinformatics advice and Simon T Cooper for assistance in the preparation of the paper. Albert Tenesa acknowledges funding from Cancer Research UK.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Rights and permissions
About this article
Cite this article
Christoforou, A., Le Hellard, S., Thomson, P. et al. Association analysis of the chromosome 4p15–p16 candidate region for bipolar disorder and schizophrenia. Mol Psychiatry 12, 1011–1025 (2007). https://doi.org/10.1038/sj.mp.4002003
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4002003
Keywords
This article is cited by
-
A higher dysregulation burden of brain DNA methylation in female patients implicated in the sex bias of Schizophrenia
Molecular Psychiatry (2023)
-
Convergence of linkage, association and GWAS findings for a candidate region for bipolar disorder and schizophrenia on chromosome 4p
Molecular Psychiatry (2011)
-
Interacting haplotypes at the NPAS3 locus alter risk of schizophrenia and bipolar disorder
Molecular Psychiatry (2009)
-
Common Variations in 4p Locus are Related to Male Completed Suicide
NeuroMolecular Medicine (2009)