Abstract
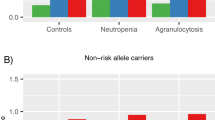
The etiology of schizophrenia is unclear, although family, twin, and linkage studies implicate genetic factors. Here, we identified adenomatous polyposis coli (APC), a tumor suppressor gene, as a risk factor for schizophrenia. We compared leukocytic gene expression patterns of six pairs of patients with schizophrenia and healthy controls by microarray. APC expression levels were significantly increased in all patients compared to healthy controls. To confirm the findings of microarray analysis, we measured expression levels of APC in the leukocytes from 30 relapse patients taking antipsychotic medication, 29 first-episode drug-naïve patients, and 30 healthy controls using real-time quantitative reverse transcription (RT)-polymerase chain reaction (PCR). APC expression levels were significantly increased in leukocytes of schizophrenics both taking and not taking antipsychotic medication and hence the increase of APC expression was not due to antipsychotic medication. APC is located at 5q21–22, which has been previously reported to be linked with schizophrenia. Further, we performed the transmission disequilibrium test (TDT) and TDT based on haplotypes to search for the association between schizophrenia and APC by examining 163 parent–offspring trios of Chinese descent. We analyzed three single-nucleotide polymorphisms (SNPs) (rs2229992, rs42427, rs465899) at the exon region of APC. TDT showed that the three SNPs are significantly associated with schizophrenia (TDT χ2=4.23, P<0.05; 4.15, P<0.05; 8.49 P<0.01, respectively; HHRRχ2=5.54, P<0.05; 4.40, P<0.05; 9.79, P<0.01, respectively). We found a significant association between the APC haplotypes from rs2229992–rs42427–rs465899 and schizophrenia (Global χ2=44.376,df=7, P<0.001). The C–A–T haplotype has a frequency of more than 57% and has a strong association with schizophrenia (χ2=15.04, P<0.001). These results indicate that the APC may be a candidate gene conferring susceptibility to schizophrenia and also may be associated with reduced vulnerability to cancer in schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Catts VS, Catts SV . Apoptosis and schizophrenia: is the tumour suppressor gene, p53, a candidate susceptibility gene? Schizophr Res 2000; 41: 405–415.
Cohen M, Dembling B, Schorling J . The association between schizophrenia and cancer: a population-based mortality study. Schizophr Res 2002; 57: 139–146.
Park JK, Lee HJ, Kim JW, Park YH, Lee SS, Chang HI et al. Differences in p53 gene polymorphisms between Korean schizophrenia and lung cancer patients. Schizophr Res 2004; 67: 71–74.
Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H et al. Identification and characterization of the familial adenomatous coli gene. Cell 1991; 66: 589–600.
Groden J, Gelbert L, Thliveris A, Nelson L, Robertson M, Joslyn G et al. Mutation analysis of patients with adenomatous polyposis: identical inactivating mutations in unrelated individuals. Am J Hum Genet 1993; 52: 263–272.
Usadel H, Brabender J, Danenberg KD, Jeronimo C, Harden S, Engles J et al. Quantitative adenomatous polyposis coli promoter methylation analysis in tumor tissue, serum, and plasma DNA of patients with lung cancer. Cancer Res 2002; 62: 371–375.
Gerstein AV, Almeida TA, Zhao G, Chess E, Shih IeM, Buhler K et al. CTNNB1 (beta-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer 2002; 34: 9–16.
Akiyama T . Wnt/beta-catenin signaling. Cytokine Growth Factor Rev 2000; 11: 273–282.
Pleasure SJ . An arrow hits the Wnt signaling pathway. Trends Neurosci 2001; 24: 69–71.
Cotter D, Kerwin R, Al-Sarraji S, Brion JP, Chadwich A, Lovestone S et al. Abnormalities of Wnt signalling in schizophrenia—evidence for neurodevelopmental abnormality. NeuroReport 1998; 9: 1379–1383.
Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA . Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 2004; 36: 131–137.
Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell 1997; 90: 895–905.
Miyaoka T, Seno H, Ishino H . Increased expression of Wnt-1 in schizophrenic brains. Schizophr Res 1999; 38: 1–6.
Beasley C, Cotter D, Khan N, Pollard C, Sheppard P, Varndell I et al. Glycogen synthase kinase-3beta immunoreactivity is reduced in the prefrontal cortex in schizophrenia. Neurosci Lett 2001; 20: 117–120.
Kozlovsky N, Belmaker RH, Agam G . GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur Neruopsychopharmacol 2002; 12: 13–25.
Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR et al. Association of the APC product with beta-catenin. Science 1993; 262: 1731–1734.
Su LK, Vogelstein B, Kinzler KW . Association of the APC tumor suppressor protein with catenins. Science 1993; 262: 1734–1737.
Vawter MP, Cannon-Spoor HE, Hemperly JJ, Hyde TM, VanderPutten DM, Kleinman JE et al. Abnormal expression of cell recognition molecules in schizophrenia. Exp Neurol 1998; 149: 424–432.
Poltorak M, Wright R, Hemperly JJ, Torrey EF, Issa F, Wyatt RJ et al. Monozygotic twins discordant for schizophrenia are discordant for N-CAM and L1 in CSF. Brain Res 1997; 751: 152–154.
Matsumine A, Ogai A, Senda T, Okumura N, Satoh K, Baeg GH et al. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science 1996; 272: 1020–1023.
Kawasaki Y, Senda T, Ishidate T, Koyama R, Morishita T, Iwayama Y et al. Asef, a link between the tumor suppressor APC and G-protein signaling. Science 2000; 289: 1194–1197.
Wang J, Jing Z, Zhang L, Zhou G, Braun J, Yao Y et al. Regulation of acetylcholine receptor clustering by the tumor suppressor APC. Nat Neurosci 2003; 6: 1017–1018.
Conrad A, Abebe T, Austin R, Forsythe S, Scheibel A . Hippocampal pyramidal cell disarray in schizophrenia as a bilateral phenomenon. Arch. Gen. Psychiatry 1991; 48: 413–417.
Cotter D, Kerwin R, Doshi B, Martin CS, Everall IP . Alteration in hippocampal non-phosphorylated MAP2 protein expression in schizophrenia. Brain Res 1997; 756: 238–246.
Mirnics K, Middleton FA, Lewis DA, Levitt P . Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci 2001; 24: 479–486.
Weinberger D . Schizophrenia, from neuropathology to neurodevelopment. Lancet 1995; 346: 552–557.
Straub RE, MacLean CJ, Ma Y, Webb BT, Myakishev MV, Harris-Kerr C et al. Genome-wide scans of three independent sets of 90 Irish multiplex schizophrenia families and follow-up of selected regions in all families provides evidence for multiple susceptibility genes. Mol Psychiatry 2002; 7: 542–559.
Straub RE, MacLean CJ, O'Neill FA, Walsh D, Kendler KS . Support for a possible schizophrenia vulnerability locus in region 5q22–31 in Irish families. Mol Psychiatry 1997; 2: 148–155.
Shawn H, Jack Y . Biological relevance of GenePix results. 2001; http://www.axon.com/MRTechnicalSupport.html.
Relative quantitation of gene expression. User Bulletin #2: ABI Prism 7700 Sequence Detection System, PE Applied Biosystems. 1997 29,30 (updated 10/2001).
Terwilliger JD, Ott J . A haplotype-based ‘haplotype relative risk’ approach to detecting allelic associations. Hum Hered 1992; 42: 337–346.
Spielman RS, McGinnis RE, Ewens WJ . Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet 1993; 52: 506–516.
Clayton D, Johns HB . Transmission/disequilibrium test for extended marker haplotypes. Am J Hum Genet 1999; 65: 1161–1169.
Gladkevich A, Kauffman HF, Korf J . Lymphocytes as a neural probe: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 559–576.
Vawter MP, Ferran E, Galke B, Cooper K, Bunney WE, Byerley W . Microarray screening of lymphocyte gene expression differences in a multiplex schizophrenia pedigree. Schizophr Res 2004; 67: 41–52.
Williams NM, O'Donovan MC, Owen MJ . Genome scans and microarrays: converging on genes for schizophrenia? Genome Biol 2002; 3: 1011.1–1011.5.
Ni X, Trakalo JM, Mundo E, Macciardi FM, Parikh S, Lee L et al. Linkage disequilibrium between dopamine D1 receptor gene (DRD1) and bipolar disorder. Biol Psychiatry 2002; 52: 1144–1150.
Acknowledgements
This research was supported by the Shanghai Mental Health Center. We thank the Health Science Center of Chinese Academy of Sciences, and Xi'an Jiaotong University. We also thank Professors SB Li, Dr TB Jin, Dr GW Xu, Ms YP Qian, and Mr DX Wang for help with data analysis and experiment, and Dr XD Wu for his helpful suggestions. Lastly, we especially thank anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, D., Jiang, K., Jiang, S. et al. The tumor suppressor adenomatous polyposis coli gene is associated with susceptibility to schizophrenia. Mol Psychiatry 10, 669–677 (2005). https://doi.org/10.1038/sj.mp.4001653
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001653
Keywords
This article is cited by
-
Ubiquitous neurocognitive dysfunction in familial adenomatous polyposis: proof-of-concept of the role of APC protein in neurocognitive function
Hereditary Cancer in Clinical Practice (2020)
-
Mortality of site-specific cancer in patients with schizophrenia: a systematic review and meta-analysis
BMC Psychiatry (2019)
-
The Interaction of TXNIP and AFq1 Genes Increases the Susceptibility of Schizophrenia
Molecular Neurobiology (2017)
-
Genome-wide copy-number variation study of psychosis in Alzheimer’s disease
Translational Psychiatry (2015)
-
Targeted deletion of the C-terminus of the mouse adenomatous polyposis coli tumor suppressor results in neurologic phenotypes related to schizophrenia
Molecular Brain (2014)