Abstract
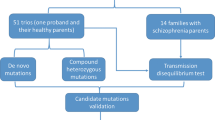
The dystrobrevin-binding protein 1 (DTNBP1) gene on chromosome 6p has emerged as a potential susceptibility gene for schizophrenia. Although a number of attempts to replicate the original association finding have been successful, they have not identified any obvious pathogenic variants or a single at risk haplotype common to all populations studied. In the present study we attempted further replication in an independent sample of 638 nuclear families from the Han Chinese population of Sichuan Province, SW China. We also examined 580 Scottish schizophrenic cases and 620 controls. We genotyped 10 single-nucleotide polymorphisms (SNPs) in DTNBP1 that were used in the original report of association, plus rs2619538 (SNP ‘A’) in the putative promoter region, which has also been associated with schizophrenia. In the Chinese trios we found that two SNPs (P1635 and P1765) were significantly overtransmitted, but with alleles opposite to those reported in the original studies. SNPs P1757 and P1765 formed a common haplotype, which also showed significant overtransmission. In the Scottish cases and controls, no individual markers were significantly associated with schizophrenia. A single haplotype, which included rs2619538 and P1583, and one rare haplotype, composed of P1320 and P1757, were significantly associated with schizophrenia, but no previously reported haplotypes were associated. Based on the data from the Chinese population, our results provide statistical support for DTNBP1 as a susceptibility gene for schizophrenia, albeit with haplotypes different from those of the original study. However, our lack of replication in the Scottish samples also indicates that caution is warranted when evaluating the robustness of the evidence for DTNBP1 as genetic risk factor for schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Accession codes
References
Cardno AG, Gottesman II . Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet 2000; 97: 12–17.
Riley BP, McGuffin P . Linkage and associated studies of schizophrenia. Am J Med Genet 2000; 97: 23–44.
Badner JA, Gershon ES . Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry 2002; 7: 405–411.
Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, Part II: Schizophrenia. Am J Hum Genet 2003; 73: 34–48.
Funke B, Finn CT, Plocik AM, Lake S, DeRosse P, Kane JM et al. Association of the DTNBP1 locus with schizophrenia in a US population. Am J Hum Genet 2004; 75: 891–898.
Kirov G, Ivanov D, Williams NM, Preece A, Nikolov I, Milev R et al. Strong evidence for association between the dystrobrevin binding protein 1 gene (DTNBP1) and schizophrenia in 488 parent-offspring trios from Bulgaria. Biol Psychiatry 2004; 55: 971–975.
Schwab SG, Knapp M, Mondabon S, Hallmayer J, Borrmann-Hassenbach M, Albus M et al. Support for association of schizophrenia with genetic variation in the 6p223 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet 2003; 72: 185–190.
Straub RE, Jiang Y, MacLean CJ, Ma Y, Webb BT, Myakishev MV et al. Genetic variation in the 6p223 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet 2002; 71: 337–348.
Tang JX, Zhou J, Fan JB, Li XW, Shi YY, Gu NF et al. Family-based association study of DTNBP1 in 6p223 and schizophrenia. Mol Psychiatry 2003; 8: 717–718.
van den Oord EJ, Sullivan PF, Jiang Y, Walsh D, O’Neill FA, Kendler KS et al. Identification of a high-risk haplotype for the dystrobrevin binding protein 1 (DTNBP1) gene in the Irish study of high-density schizophrenia families. Mol Psychiatry 2003; 8: 499–510.
Williams NM, Preece A, Morris DW, Spurlock G, Bray NJ, Stephens M et al. Identification in 2 independent samples of a novel schizophrenia risk haplotype of the dystrobrevin binding protein gene (DTNBP1). Arch Gen Psychiatry 2004; 61: 336–344.
Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ . Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem 2001; 276: 24232–24241.
Li W, Zhang Q, Oiso N, Novak EK, Gautam R, O’Brien EP et al. Hermansky–Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1). Nat Genet 2003; 35: 84–89.
Sillitoe RV, Benson MA, Blake DJ, Hawkes R . Abnormal dysbindin expression in cerebellar mossy fiber synapses in the mdx mouse model of Duchenne muscular dystrophy. J Neurosci. 2003; 23: 6576–6585.
Falcon-Perez JM, Starcevic M, Gautam R, Dell’Angelica EC . BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J Biol Chem 2002; 277: 28191–28199.
Numakawa T, Yagasaki Y, Ishimoto T, Okada T, Suzuki T, Iwata N et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet 2004; 13: 2699–2708.
Straub RE, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F et al. A potential vulnerability locus for schizophrenia on chromosome 6p24–22: evidence for genetic heterogeneity. Nat Genet 1995; 11: 287–293.
Wang S, Sun CE, Walczak CA, Ziegle JS, Kipps BR, Goldin LR, et al. Evidence for a susceptibility locus for schizophrenia on chromosome 6pter–p22. Nat Genet 1995; 10: 41–46.
Van Den Bogaert A, Schumacher J, Schulze TG, Otte AC, Ohlraun S, Kovalenko S et al. The DTNBP1 (dysbindin) gene contributes to schizophrenia, depending on family history of the disease. Am J Hum Genet 2003; 73: 1438–1443.
Morris DW, McGhee KA, Schwaiger S, Scully P, Quinn J, Meagher D et al. No evidence for association of the dysbindin gene [DTNBP1] with schizophrenia in an Irish population-based study. Schizophr Res 2003; 60: 167–172.
Kohn Y, Danilovich E, Filon D, Oppenheim A, Karni O, Kanyas K et al. Linkage disequlibrium in the DTNBP1 (dysbindin) gene region and on chromosome 1p36 among psychotic patients from a genetic isolate in Israel: findings from identity by descent haplotype sharing analysis. Am J Med Genet 2004; 128B: 65–70.
Talbot K, Eidem WL, Tinsley CL, Benson MA, Thompson EW, Smith RJ . Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004; 113: 1353–1363.
Weickert CS, Straub RE, McClintock BW, Matsumoto M, Hashimoto R, Hyde TM et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry 2004; 61: 544–555.
Cai G, Li T, Deng H, Zhao J, Hu X, Murray RM et al. Affected sibling pair linkage analysis of qualitative and quantitative traits for schizophrenia on chromosome 22 in a Chinese population. Am J Med Genet 2001; 105: 321–327.
Li T, Ball D, Zhao J, Murray RM, Liu X, Sham PC et al. Family-based linkage disequilibrium mapping using SNP marker haplotypes: application to a potential locus for schizophrenia at chromosome 22q11. Mol Psychiatry 2000; 5: 77–84.
Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet 2003; 72: 83–87.
Slatkin M, Excoffier L . Testing for linkage disequilibrium in genotypic data using the Expectation-Maximization algorithm. Heredity 1996; 76 (Part 4): 377–383.
Hill WG, Weir BS . Maximum-likelihood estimation of gene location by linkage disequilibrium. Am J Hum Genet 1994; 54: 705–714.
Dudbridge F . Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003; 25: 115–121.
Russell RK, Wilson DC, Satsangi J . Unravelling the complex genetics of inflammatory bowel disease. Arch Dis Child 2004; 89: 598–603.
Helgason A, Yngvadottir B, Hrafnkelsson B, Gulcher J, Stefansson K . An Icelandic example of the impact of population structure on association studies. Nat Genet 2005; 37: 90–95.
Akey JM, Zhang K, Xiong M, Doris P, Jin L . The effect that genotyping errors have on the robustness of common linkage-disequilibrium measures. Am J Hum Genet. 2001; 68: 1447–1456.
Acknowledgements
This work was partly funded by National Nature Science Foundation of China (TL), NARSAD (TL), Schizophrenia Research Fund (TL and DAC), the Psychiatry Research Trust (TL, RMM, DAC) and the Wellcome Trust for an International Collaborative award (TL, XS, DAC, XL). GSK also helped fund Scottish sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, T., Zhang, F., Liu, X. et al. Identifying potential risk haplotypes for schizophrenia at the DTNBP1 locus in Han Chinese and Scottish populations. Mol Psychiatry 10, 1037–1044 (2005). https://doi.org/10.1038/sj.mp.4001718
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001718
Keywords
This article is cited by
-
Increased dysbindin-1B isoform expression in schizophrenia and its propensity in aggresome formation
Cell Discovery (2015)
-
Korrelation zwischen Risikogenvarianten für Schizophrenie und Hirnstrukturanomalien
Der Nervenarzt (2009)
-
Recent Progress in the Research Field of Neuropharmacology in China
Cellular and Molecular Neurobiology (2008)
-
Failure to confirm allelic and haplotypic association between markers at the chromosome 6p22.3 dystrobrevin-binding protein 1 (DTNBP1) locus and schizophrenia
Behavioral and Brain Functions (2007)
-
Molecular genetic studies of schizophrenia
European Journal of Human Genetics (2006)