Abstract
Lymph node size affects lymph node retrieval in surgical specimen and is used as criterion for pre-operative radiological estimation of metastatic disease. However, factors determining lymph node size remain to be established. Therefore, the association between lymph node size and presence of metastatic cancer deposits as well as different primary tumor characteristics was analyzed in a prospective cross-sectional study. Visible and palpable nodes were harvested, and conventional histology, immunohistochemistry, and molecular analysis were performed. The study cohort comprised 148 patients (median age 69 years, range 36–92). Lymph node dissection rendered 4167 nodes. Mean lymph node count was 28 (median 26, range 9–67). Metastatic disease was detected in 320 (8%) nodes and was associated with lymph node size (P<0.001). Positive nodes measuring ≤2 mm caused upstaging within the N category in one third of cases, but did not identify patients as node-positive as all patients also had positive larger nodes. Large tumor size (P=0.001), right tumor location (P<0.001), and deep tumor penetration (P=0.024) were all independently associated with lymph node size, whereas high lymphocytic antitumor reaction just missed statistical significance (P=0.053) in multivariable analysis. Microsatellite instability had no influence on lymph node size when analysis was restricted to right-sided tumors. In conclusion, analysis of small lymph nodes may lead to upstaging within the N category, but they do not identify a patient as node-positive and do therefore not influence clinical decision-making in the adjuvant setting. The majority of enlarged lymph nodes, including those measuring >1 cm, are not involved by cancer. Different tumor characteristics, such as large primary tumor size, right tumor location, and deep tumor penetration are independently associated with lymph node size and need to be considered when interpreting enlarged nodes detected by radiological imaging.
Similar content being viewed by others
Main
Outcome prediction based on tumor stage reflected by the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) tumor node metastasis (TNM) system is the mainstay of clinical decision-making in patients with colorectal cancer.1, 2
The indication for adjuvant therapy is guided (mainly) by the presence of lymph node metastasis in the resection specimen as documented in the pathology report.3 The extent of lymph node retrieval from the resection specimen therefore affects staging accuracy and has been associated with outcome.4, 5 Lymph node retrieval is dependent on lymph node size,6, 7 which may be explained by easier identification of larger nodes within the mesenterial fatty tissue.
In locally advanced rectal cancer, improved local control and favorable toxicity have been observed in patients, who received pre-operative radiochemotherapy as compared with postoperative treatment.8 Pre-operative assessment of nodal status by ultrasound or radiological imaging, mainly based on the size of lymph nodes, contributes to decision making, causing huge implications of either understaging or overstaging of lymph node involvement. For instance, overstaging was reported in 18% of patients in the German rectal cancer study.8 Another study reported that 22% of patients undergoing pre-operative radiochemotherapy, clinically staged T3 N0, had undetected positive lymph nodes in their resection specimens.9
These data suggest that lymph node size may not be a good marker for the diagnosis of lymph node metastasis, as the relationship between lymph node size and lymph node involvement by tumor cells is inaccurate. However, the factors determining lymph node size apart from metastatic disease are largely unknown. Moreover, the impact of small lymph nodes on staging is controversial.
Therefore, we conducted a prospective cross-sectional study to investigate the relationship between the size of lymph nodes and involvement by metastatic cancer tissue. In consecutively recruited patients, we analyzed a large number of tumor-associated parameters, such as tumor size, grade, and location as well as the extent of tumor necrosis, peritumoral inflammation, lymphovascular, and perineural invasion as well as tumor budding, to identify those that might, apart from metastatic cancer spread, cause lymph node enlargement.
Materials and methods
We conducted a prospective cross-sectional study to investigate the relationship between lymph node size and presence of metastatic cancer deposits as well as different primary tumor characteristics. Data will be presented following the STROBE Statement aimed at strengthening the reporting of observational studies.10
The investigation was carried out in accordance with the Declaration of Helsinki. The study was approved by the Institutional Review Board of the Medical University of Graz, Austria.
Study Population
Between January 2011 and December 2013, 175 patients with colorectal cancer underwent surgical treatment at the Department of Surgery, Krankenhaus der Barmherzigen Brüder, St Veit/Glan, Austria, and were prospectively enrolled in the investigation. To control for procedure-related factors with impact on the extent of surgical lymph node dissection, the majority of resections were performed by two surgeons only and the remaining patients operated by other surgeons applying the same technique under supervision.
Patients with rectal cancer who underwent neoadjuvant chemoradiation (n=24) were excluded from analysis as this procedure is known to affect both lymph node yield and lymph node size.2 In addition, we excluded patients with multiple (synchronous) primary colorectal tumors (n=3) as correlation between primary tumor and lymph node characteristics is not feasible in these cases. Thus, the final (study) cohort comprised 148 patients, including 90 males and 58 females (ratio 1.6:1) with mean age of 68 years (median 69, range 36–92).
Macroscopic Evaluation of Resection Specimens
Immediately after surgery, the resection specimens were submitted to 10% neutral buffered formalin and fixed for a minimum of 24 h. The macroscopic evaluation, including lymph node recovery was performed by a single gastrointestinal pathologist (CL) in 127 (86%) cases. The remaining 21 specimens were examined by two other experienced consultants.
The maximum diameter of the primary tumor was measured and the depth of penetration into the bowel wall recorded. Lymph nodes were manually dissected according to a standardized protocol. Specifically, lymph nodes were dissected by palpating along the major vessels, followed by transverse sectioning of the mesenterial fatty tissue, which was visually inspected and thoroughly palpated for further lymph nodes. No ancillary techniques, such as fat clearing (eg, acetone elution with or without fat compression) or methylene blue injection were applied in this study.
Tumors located in the cecum to transverse colon were recorded as right-sided cancers and tumors located in the left colonic flexure to rectosigmoid junction as left-sided cancers. All lesions located within 16 cm of the anal verge were recorded as rectal cancers.
Histology
Paraffin-embedded tumor and lymph node tissues were cut as 4 μm thick sections and stained with hematoxylin and eosin (H&E) for histological examination.
The maximum diameter of each recovered lymph node was measured in millimeters from the original slides by one examiner (OR), using the method described by Murphy et al.11 All nodes were analyzed microscopically for metastatic infiltration assessing a minimum of two levels. The histology of all primary tumors was evaluated by one pathologist (CL), blinded to lymph node data. Tumor stage was assessed according to the 7th edition of the AJCC/UICC TNM classification.12 Tumor grading was performed according to WHO guidelines, assessing the extent of glandular appearance.13
The (overall) inflammation at the invasive margin was estimated using a four-degree scale as described by Klintrup et al.14 Following the method described by Ogino et al.15 four components of lymphocytic antitumor reactions were examined, ie, Crohn's-like lymphoid reaction, peritumoral lymphocytic reaction, intratumoral periglandular reaction, and tumor-infiltrating lymphocytes. Each of the four lymphocytic reaction components was scored as 0 (absent), 1+ (mild), 2+ (moderate), or 3+ (marked). The overall lymphocytic reaction score (0–12) was the sum of the scores for the four reaction components. In addition, we evaluated the extent of histological tumor necrosis using a four-degree scale as previously reported.16
Lymphovascular invasion, including both venous and lymphatic invasion, was considered positive when tumor cells or tumor cell thrombi were observed within an endothelium-lined space. Special care was taken to differentiate endothelial cells from retraction artifacts lined by fibroblasts. Immunohistochemistry for endothelial cells was not routinely done, in keeping with standard practice.17 Perineural invasion was defined as presence of tumor cells within any layer of the nerve sheath or tumor in the perineural space that involved at least one third of the nerve circumference.18 The extent of tumor budding (presence of isolated single cells or small clusters of tumor cells in the stroma at the invasive tumor margin) was assessed on H&E-stained slides in a field in which budding intensity was maximal.19 The number of budding foci was scored as low grade (<10 budding foci) or high grade (>9 budding foci).20
Immunohistochemistry
Four monoclonal antibodies, which are directed against different mismatch repair (MMR) proteins, were used in the investigation: MLH1 (clone G168-15, 1:50; Biocare, Concorde, CA, USA), MSH2 (clone G219-1129, ready to use; Ventana, Tucson, AZ, USA), MSH6 (clone BC-44; 1:50; Biocare) and PMS2 (clone MRQ-28, 1:50; Cell Marque, Rocklin, CA, USA). For a tumor to be considered MMR-proficient, immunoreactivity for MLH1, MSH2, MSH6, and PMS2 was required, whereas loss of at least one of the four markers characterized MMR-deficient tumors. All MMR-deficient tumors were subsequently tested for microsatellite instability (MSI).
Molecular Analysis
DNA was extracted from formalin-fixed paraffin-embedded tissues using the Qiagen QIAmp DNA Mini Kit (Qiagen, Hilden, Germany). MSI was investigated using the Promega Microsatellite Analysis System version 1.2 (Promega, Mannheim, Germany), a PCR multiplex system using five mononucleotide markers (BAT-25, BAT-26, NR-21, NR-24, MONO-27) to determine MSI and two pentanucleotide repeat markers (Penta C and Penta D) for internal control. PCR products were separated by capillary electrophoresis using an ABI Prism 3100 genetic analyzer (Applied Biosystems, Vienna, Austria). MSI at two mononucleotide loci was reported as MSI-high, instability at one locus as MSI-low and no instability at any of the loci tested as microsatellite stable (MSS).
Statistical Analysis
Categorical variables are presented as absolute and relative frequencies, numerical variables as means and medians as well as ranges. Differences in categorical variables were examined using the χ2-test or Fisher’s exact test, as appropriate. Differences in numerical variables were assessed using Student’s t-test. Receiver-operator characteristic (ROC) curve was performed in order to identify a threshold that provides the best discrimination (highest sum of sensitivity and specificity) between lymph node negative and positive tumors. Binary logistic regression was applied to assess the influence of primary tumor characteristics on lymph node size. Herein, the Hosmer–Lemeshow test was used to assess goodness of fit of the model. Results are presented as adjusted hazards ratios and 95% confidence intervals. All statistical operations were performed using SPSS statistics 20 (IBM, Armonk, New York, USA). P-values were two-sided and values <0.05 were considered statistically significant.
Results
Primary Tumor Characteristics
Tumors were located in the cecum in 18 (12%), in the ascending colon in 24 (16%), at the hepatic flexure in 14 (9%), in the transverse colon in 6 (4%), at the splenic flexure in 3 (2%), in the descending colon in 6 (4%), in the sigmoid colon in 42 (28%), at the rectosigmoid junction in 11 (7%), and in the rectum in 24 (16%) patients, respectively.
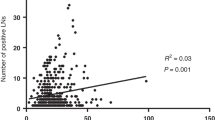
Mean tumor size was 4 cm (median 3.8, range 0.5–17). Fifteen (10%) tumors were classified pT1, 24 (16%) pT2, 68 (46%) pT3, 31 (21%) pT4a, and 10 (7%) pT4b, respectively. Lymph node metastasis was detected in 61 (41%) cases, with 18 (12%) and 15 (10%) cases classified N1a and N1b as well as 15 (10%) and 13 (9%) cases classified N2a and N2b, respectively. Mean number of positive nodes was 5.3 (median 3, range 1–32). Tumor grades were G1 in 38 (26%), G2 in 60 (41%), and G3 in 50 (34%) cases. Lymphovascular invasion and perineural invasion were noted in 76 (51%) and 28 (19%) cases, respectively. Tumors of 60 patients (41%) exhibited high-grade tumor budding.
Lymph Node Size is Related to Tumor Location and Presence of Lymph Node Metastasis
Lymph node dissection rendered 4167 nodes. Mean lymph node count was 28 (median 26, range 9–67). In 145 (98%) cases, 12 or more nodes were recovered. Mean lymph node size was 3.8±0.8 mm (median 3, range 1–24). Mean lymph node size from right-sided cancers was larger than that of left-sided cancers (4.2±0.8 mm vs 3.5±0.8 mm; P<0.001).
Metastatic cancer spread was detected in 320 (8%) lymph nodes. Mean size of positive nodes was 5.6±1.9 mm, compared with 3.6±0.8 mm in negative nodes (P<0.001; Figure 1a). Mean lymph node size in N-positive tumors was 4.0±0.9 mm, compared with 3.6±0.8 mm in N-negative tumors (P=0.008; Figure 1b).
Lymph node size was related to the presence of metastatic disease, and the larger the node, the higher the risk of metastatic infiltration. Metastasis can be identified in lymph nodes as small as 1 mm. It is of note that 74% of lymph nodes larger than 10 mm were free of cancer (Table 1). All 18 patients with positive nodes measuring 1 or 2 mm had metastases in larger nodes. The identification of positive nodes measuring 1 or 2 mm caused a change in N classification (upstaging) in six (33%) cases. Twenty-seven patients harbored positive nodes measuring 3 mm in largest diameter. The identification of these nodes caused upstaging in 13 (48%) cases. In three cases, the positive 3 mm nodes were the only positive nodes, classifying these patients for AJCC/UICC stage III disease.
Primary Tumor Characteristics are Related to the Presence of Lymph Node Metastasis
Several primary tumor characteristics were related to the presence of lymph node metastasis. Specifically, increasing T classification, tumor size, grade, presence of tumor necrosis, lymphovascular and perineural invasion, as well as high-grade tumor budding were positively associated with metastatic disease, whereas increasing lymphocytic antitumor reaction was negatively associated with the presence of lymph node metastasis (Table 2).
Primary Tumor Characteristics are Related to the Size of Regional Lymph Nodes
In ROC analysis (area under the curve of 0.642, 95% CI 0.53–0.71; P=0.011) including the number of lymph nodes with a size of ≥5 mm, a cutoff value of at least five lymph nodes showed highest sensitivity (85%) and specificity (32%) regarding discrimination between lymph node negative and positive tumors. Therefore this cutoff value was used for further analyses.
Several primary tumor characteristics were related to the presence of at least five nodes ≥5 mm (Table 3). Parameters that were significantly associated were included in multivariable analysis (binary logistic regression), which identified large tumor size, high T classification and right tumor location as independent parameters. High lymphocytic antitumor reaction had the highest adjusted hazard ratio, but analysis just missed statistical significance (P=0.053). Notably, nodal metastasis was not found to be independently associated with the presence of at least five nodes ≥5 mm (Table 4). Hosmer–Lemeshow’s measure reveals an adequate fit of the model (χ2(8)=10.86; P=0.21).
As MSI cancers are known to show marked intra- and peritumoral inflammation21 we speculated that lymph nodes in MSI cancers might be larger than those in MSS cancers. In all, 20 (14%) tumors were MMR-deficient, with loss of MLH1 (and PMS2) expression as the most frequent finding. All MMR-deficient tumors were MSI-high on molecular analysis, and all but three MMR-deficient tumors were right-sided tumors. Investigating all 148 cancers, mean lymph node size in MSI cancers was 4.2±0.8 mm, compared with 3.7±0.8 mm in MSS cancers (P=0.023). Similar data were obtained, when analyses were restricted to positive or negative nodes (data not shown). As MSI cancers occurred predominantly on the right side and right-sided cancer specimens did per se harbor larger nodes (compare above), we restricted the comparison of MSI and MSS cancers with right-sided tumors. In this subset, no significant difference in lymph node size was observed (4.2±0.7 mm in MSI cancers, compared with 4.1±0.8 mm in MSS cancers; P=0.62).
Discussion
In colorectal cancer, the lymph node status is a prognostic parameter of eminent clinical importance. Positive nodes guide clinical decision-making by selecting AJCC/UICC stage III colon cancer patients for adjuvant chemotherapy.3 In patients with rectal cancer, the decision for neoadjuvant treatment is mainly based on the depth of tumor penetration, but also on clinical lymph node assessment.22, 23 Both, pre-operative clinical lymph node assessment and (post-)operative lymph node harvest are influenced by the size of lymph nodes. However, the relationship between lymph node size and lymph node involvement is inconsistent.
In our investigation, nodes harboring metastatic cancer deposits were significantly larger than uninvolved nodes. The size of positive nodes was 5.6±1.9 mm, compared with 3.6±0.8 mm for negative nodes. This is in accordance with previous studies.6, 24, 25 For instance, Mönig et al.24 reported a mean diameter of 5.9 mm for involved nodes compared with 3.9 mm for uninvolved nodes. In that study, metastases occurred in 36.5% of nodes measuring >5 mm compared with 13.3% of nodes measuring ≤5 mm.
We found a nearly linear relationship between lymph node size and presence of metastatic cancer spread, as shown in Table 1. Other authors have noted similar associations. In the study by Märkl et al.26 almost half of the 305 detected metastases were found in lymph nodes with diameters ≤5 mm, demonstrating that metastatic disease can be identified already in very small nodes. It is of note that Sloothaak et al.6 failed to identify a relevant cutoff point to predict metastatic involvement applying ROC curve analysis.
In our study, 1 of 266 (0.4%) nodes measuring 1 mm and 29 of 983 (3%) nodes measuring 2 mm harbored metastatic cancer deposits. Accordingly, Märkl et al.26 reported that only 2 of 413 (0.5%) lymph nodes in the 1 mm category were involved by cancer. Sloothaak et al.6 found that nodes smaller than 3 mm were positive in 8% patients, but were the only reason for upstaging in only two cases. In our investigation, positive nodes measuring ≤2 mm caused upstaging within the N category in one third of cases, but they did not identify a patient as node-positive (N0→N1/N2), as all patients with metastases in these small nodes had positive larger nodes. Hence, lymph node size is associated with lymph node involvement by tumor, but also small lymph nodes may contain metastatic deposits. It is clinically relevant that patients with small lymph node metastases usually have metastases in accompanying larger nodes.
This imposes the question whether small lymph nodes need to be sampled at all. This question cannot be answered definitively, but a clue may be obtained from studies investigating the benefit of techniques that increase the lymph node harvest in cancer specimens, such as fat clearing methods, methylene blue-assisted lymph node dissection, or acetone elution with subsequent compression of adipose tissue (‘acetone compression’).2 These techniques have been shown to result in increased lymph node counts compared with conventional dissection.27, 28 However, recently published data indicate that the application of these techniques is not associated with an increased detection of lymph node metastases.28
Our finding that only 21 of 82 (26%) nodes >10 mm had metastatic disease is in the line with previous data from Märkl et al.26 who observed metastatic disease in 27% in this subgroup. Thus, the majority of large nodes are not involved by cancer. However, the factors determining lymph node size in the absence of metastatic disease, ie, the factors that derogate the clinical value of lymph node size as predictor of metastatic disease have been largely unknown. We identified large tumor size, deep tumor penetration (high T category) and right tumor location as independent predictors of large regional lymph nodes. Notably, the presence of nodal cancer spread was not significantly associated with the presence of enlarged lymph nodes in multivariable analysis.
Large tumor size and/or deep tumor penetration are expected to provide a more intense antigenic immune challenge to the draining lymph nodes, which may result in nodal hyperplasia, causing reactive enlargement of regional lymph nodes.29 Our study, which analyzed different types of intra- and peritumoral inflammation, supports this hypothesis: high lymphocytic antitumor reaction markedly increased the likelihood of enlarged lymph nodes, but this marker just missed statistical significance in multivariable analysis. In contrast, the overall inflammatory cell reaction, which in addition to lymphocytes includes peritumoral neutrophils and other inflammatory cells, does not seem to have a relevant effect on lymph node size. To the best of our knowledge, there is until now only one study available that investigated the influence of different tumor characteristics on the size of recovered lymph nodes. Murphy et al.11 identified stromal lymphoid infiltrate, peritumoral lymphoid aggregates (Crohn's-like lymphoid reaction) and tumor growth pattern (circumscribed vs infiltrative) as significant parameters. This study, however, is hampered by poor lymph node recovery (mean lymph node count 12.4; median 11, range 1–33), retrospective nature and lack of adjustment by multivariable analysis.
The possible influence of intra- and peritumoral lymphocytic inflammation deserves further attention. As MSI cancers are known to show marked intra- and peritumoral lymphocytic inflammation,21 we speculated that lymph nodes in MSI cancers might be larger than those in MSS cancers. Interestingly, a high number of isolated lymph nodes in AJCC/UICC stage I or II colorectal cancer has been associated with the MSI phenotype.30 This may be due to reactive enlargement of nodes in the mesentery, which may makes them easier to find. In our study, the mean size of lymph nodes in MSI cancers was significantly larger than that of MSS cancers. MSI cancers, however, mainly occur proximal to the splenic flexure 31 and right-sided tumors do per se harbor larger nodes. This observation is in accordance with the notion that the number of retrieved nodes is usually larger in resection specimens of right-sided cancers compared with left-sided cancers.32 Therefore, in a second step, we restricted analysis to right-sided tumors. In this subset no significant difference in lymph node size was observed, when comparing MSI with MSS cancers.
How might this study ultimately affect our daily practice and what is the clinical application? First, positive nodes ≤2 mm do not identify a patient as node-positive; they do therefore not influence treatment decisions in the adjuvant setting. More studies are needed to confirm our results before a general recommendation to disregard nodes of this size can be given. But it is clear, more nodes do not necessarily imply more clinically relevant information. Second, the majority of large nodes, including those larger than 10 mm, are not involved by cancer. Thus, presence of large lymph nodes does not necessarily mean presence of lymph nodes involved by cancer. In addition, we identified several tumor characteristics, such as large tumor size, deep tumor penetration and right tumor location that were all independently associated with lymph node size. The members of the multidisciplinary team should be aware of these associations, in particular when decision for neoadjuvant treatment is based upon the clinical N category, ie, pre-operative imaging of peritumoural lymph nodes, alone.
Our study has strengths and limitations. Apart from the prospective design and the standardized study protocol, strengths include the high lymph node recovery and the different efforts that were undertaken to control the quality of the dataset. The number of involved surgeons was kept to a minimum, gross examination of the resection specimens was done by only one pathologist in the vast majority of cases, and only one examiner measured the size of the recovered lymph nodes. Nevertheless, our study has limitations. Thus, although our study went over three years, the number of cases that were eligible for analysis was limited. This had impact on the informative value of subgroup analyses, in particular the analysis of MSI cancers.
In conclusion, small lymph nodes, including those measuring 1–2 mm, are less likely to harbor metastatic cancer spread. Positive small nodes cause upstaging within the N category in one third of cases, but they do not identify a patient as node-positive. The majority of large lymph nodes, including those measuring >10 mm, are not involved by cancer. Large tumor size, deep tumor penetration and right tumor location are independently associated with the presence of enlarged lymph nodes. High lymphocytic antitumor reaction markedly increased the likelihood of enlarged lymph nodes, but this marker just missed statistical significance in multivariable analysis. These factors need to be considered when interpreting the clinical significance of enlarged nodes detected by pre-operative imaging.
Dedication
This article is dedicated to the memory of a dear colleague, Peter Rehak, who died of cancer in 2015. Peter Rehak was an extraordinary colleague and was involved in this investigation from its very beginning. His charming persona and dedication to medical research are sorely missed.
References
Washington MK . Colorectal carcinoma: selected issues in pathologic examination and staging and determination of prognostic factors. Arch Pathol Lab Med 2008; 132: 1600–1607.
Resch A . Lymph node staging in colorectal cancer: Old controversies and recent advances. World J Gastroenterol 2013; 19: 8515–8526.
Benson AB, Arnoletti JP, Bekaii-Saab T et al, Colon cancer. J Natl Compr Canc Netw 2011; 9: 1238–1290.
Destri GL, Di Carlo I, Scilletta R et al, Colorectal cancer and lymph nodes: the obsession with the number 12. World J Gastroenterol 2014; 20: 1951–1960.
McDonald JR . Lymph node harvest in colon and rectal cancer: current considerations. World J Gastrointest Surg 2012; 4: 9–19.
Sloothaak DAM, Grewal S, Doornewaard H et al, Lymph node size as a predictor of lymphatic staging in colonic cancer. Br J Surg 2014; 101: 701–706.
Okada K, Sadahiro S, Suzuki T et al, The size of retrieved lymph nodes correlates with the number of retrieved lymph nodes and is an independent prognostic factor in patients with stage II colon cancer. Int J Colorectal Dis 2015; 30: 1685–1693.
Sauer R, Becker H, Hohenberger W et al, Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004; 351: 1731–1740.
Guillem JG, Diaz-Gonzalez JA, Minsky BD et al, cT3N0 rectal cancer: potential overtreatment with preoperative chemoradiotherapy is warranted. J Clin Oncol 2008; 26: 368–373.
von Elm E, Altman DG, Egger M et al, The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology 2007; 18: 800–804.
Murphy J, Pocard M, Jass JR et al, Number and size of lymph nodes recovered from dukes B rectal cancers: correlation with prognosis and histologic antitumor immune response. Dis Colon Rectum 2007; 50: 1526–1534.
Sobin LH, Gospodarowicz MK, Wittekind C (eds). International Union Against Cancer TNM Classification of Malignant Tumours, 7th edn. Wiley-Blackwell: West-Sussex, 2009, pp 100–105.
Hamilton SR, Bosman FT, Boffetta P, Ilyas M, Morreau H, Nakamura SI, Quirke P, Riboli E, Sobin LH . Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND. (eds). WHO classification of tumours of the digestive system, 4th edn. IARC: Lyon, 2010, pp 134–146.
Klintrup K, Mäkinen JM, Kauppila S et al, Inflammation and prognosis in colorectal cancer. Eur J Cancer 2005; 41: 2645–2654.
Ogino S, Nosho K, Irahara N et al, Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res 2009; 15: 6412–6420.
Pollheimer MJ, Kornprat P, Lindtner RA et al, Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol 2010; 41: 1749–1757.
Betge J, Langner C . Vascular invasion, perineural invasion, and tumour budding: predictors of outcome in colorectal cancer. Acta Gastroenterol Belg 2011; 74: 516–529.
Liebig C, Ayala G, Wilks J et al, Perineural invasion is an independent predictor of outcome in colorectalcancer. J Clin Oncol 2009; 27: 5131–5137.
Ueno H, Murphy J, Jass JR et al, Tumour “budding” as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 2002; 40: 127–132.
Betge J, Kornprat P, Pollheimer MJ et al, Tumor budding is an independent predictor of outcome in AJCC/UICC stage II colorectal cancer. Ann Surg Oncol 2012; 19: 3706–3712.
Greenson JK, Huang S-C, Herron C et al, Pathologic predictors of microsatellite instability in colorectalcancer. Am J Surg Pathol 2009; 33: 126–133.
Glimelius B, Pahlman L, Cervantes A. ESMO Guidelines Working Group. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21: 82–86.
Engstrom PF, Arnoletti JP, Benson AB et al, NCCN clinical practice guidelines in oncology: rectal cancer. J Natl Compr Cancer Netw 2009; 7: 838–881.
Mönig SP, Baldus SE, Zirbes TK et al, Lymph node size and metastatic infiltration in colon cancer. Ann Surg Oncol 1999; 6: 579–581.
Wong JH, Severino R, Honnebier MB et al, Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol 1999; 17: 2896–2900.
Märkl B, Rößle J, Arnholdt HM et al, The clinical significance of lymph node size in colon cancer. Mod Pathol 2012; 25: 1413–1422.
Märkl B, Kerwel TG, Wagner T et al, Methylene blue injection into the rectal artery as a simple method to improve lymph node harvest in rectal cancer. Mod Pathol 2007; 20: 797–801.
Gehoff A, Basten O, Sprenger T et al, Optimal lymph node harvest in rectal cancer (UICC stages II and III) after preoperative 5-FU-based radiochemotherapy. Acetone compression is a new and highly efficient method. Am J Surg Pathol 2012; 36: 202–213.
Wright FC, Law CHL, Last L et al, Lymph node retrieval and assessment in stage II colorectal cancer: a population-based study. Ann Surg Oncol 2003; 10: 903–909.
Eveno C, Nemeth J, Soliman H et al, Association between a high number of isolated lymph nodes in T1 to T4 N0M0 colorectal cancer and the microsatellite instability phenotype. Arch Surg 2010; 145: 12–17.
Thibodeau SN, Bren G, Schaid D . Microsatellite instability in cancer of the proximal colon. Science 1993; 260: 816–819.
Gelos M, Gelhaus J, Mehnert P et al, Factors influencing lymph node harvest in colorectal surgery. Int J Colorectal Dis 2008; 23: 53–59.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rössler, O., Betge, J., Harbaum, L. et al. Tumor size, tumor location, and antitumor inflammatory response are associated with lymph node size in colorectal cancer patients. Mod Pathol 30, 897–904 (2017). https://doi.org/10.1038/modpathol.2016.227
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2016.227
This article is cited by
-
The effect of preoperative endoscopic tattooing using India ink on lymph node yield in laparoscopic colectomy for stage I right-sided colon cancer
International Journal of Colorectal Disease (2023)
-
Clinical and Pathologic Predictors of Tumor Deposits in Colorectal Cancer
Journal of Gastrointestinal Cancer (2023)
-
Number of negative lymph nodes with a positive impact on survival of stage III colon cancer; a retrospective observation study for right side and left side colon
BMC Cancer (2022)
-
A greater lymph node yield is required during pathological examination in microsatellite instability-high gastric cancer
BMC Cancer (2021)
-
Rectal cancer: a methodological approach to matching PET/MRI to histopathology
Cancer Imaging (2020)