Abstract
Cellular neurothekeoma is a frequent source of diagnostic difficulty. In order to gain more insight into the range of histologic features of cellular neurothekeoma, we examined all cases from our institution, with a focus on describing atypical histologic features. Cases with sufficient histologic material for evaluation were retrieved. Cases were analyzed for demographics, growth pattern, myxoid stroma, cytologic atypia, mitotic rate, perineural invasion, and other histologic features. The 37 patients (16 M; 21 F) had a mean age of 31.0 years (range: 4–89). Tumors involved the head and neck (n=16), arms (n=11), trunk and shoulders (n=8), and foot (n=2). All cases had at least focal nesting of epithelioid to spindled tumors cells characteristic of cellular neurothekeoma. In many, alternate growth patterns were present and represented the dominant pattern in some. These patterns included fascicular (n=9), sheet-like (n=6), and corded (n=4). Myxoid stroma was present in 14 and was prominent in 5. Cytologic atypia was present in 19 patients, with 3 having severe atypia. Mean mitotic rate was 2.0/mm2 (range 0–10 per mm2). Neurotropism was seen in four cases. Other unusual features included collagen trapping, giant cells, hemorrhage, lymphocytic cuffing, chondroid stroma, and cellular vacuolization. Cellular neurothekeoma has a wider range of features than is commonly recognized. The presence of nests of epithelioid tumor cells with characteristic cytologic features, no matter how focal, is a clue to the diagnosis.
Similar content being viewed by others
Main
Cellular neurothekeoma, formerly considered a nerve sheath tumor, is a dermal neoplasm of presumed fibrohistiocytic lineage.1, 2, 3 They typically occur on the head, neck, and upper body of young adults, with a slight female predominance.2, 3, 4 The classic histology of cellular neurothekeoma is of a dermal-based lesion with a lobular growth pattern that forms nests with a swirling to slightly fascicular growth pattern. The tumor cells are epithelioid to spindled with abundant pale eosinophilic cytoplasm, oval nuclei with pinpoint nucleoli. Although most cellular neurothekeomas display these characteristic histologic features, atypical features have been described, including prominent myxoid stroma, cytologic atypia, infiltrative growth, vascular invasion, perineural invasion, fascicular and plexiform growth patterns, and dense hyalinized stroma.3, 4, 5, 6, 7, 8, 9 When atypical features are present, the diagnosis of cellular neurothekeoma can be challenging. For this reason, cellular neurothekeoma with atypical features is a relatively frequent source of referral to our consultation practice. In order to gain more insight into the range of histologic features of cellular neurothekeoma, we examined a large series from our practice with a focus on describing unusual features.
Materials and methods
We retrieved all cases of cellular neurothekeoma from 1980 to 2012 with sufficient histologic material. We recorded the demographic features and reviewed all slides including H&E-stained sections and immunohistochemical stains. Cases were analyzed for a variety of histologic features such as growth pattern, presence of myxoid stroma, cytologic atypia, mitotic rate, perineural invasion, and vascular invasion. The degree of myxoid stoma was classified as absent, mild (10–25%), or prominent (>25%) The cytologic atypia and pleomorphism were classified as mild, moderate, or severe. Mitotic rate was calculated using the hot spot method per square millimeter similar to the technique employed in the evaluation of melanomas. Any other atypical features were noted. Results of the immunohistochemical stains were abstracted from reports and, when available, re-reviewed. Reviewed immunohistochemical stains were scored as negative (<5% positivity), weakly positive (5–25% positivity), or positive (>25% positivity).
Results
After cases with insufficient material were excluded, 37 cases met selection criteria. The tumors were more common in young adults with a mean age of 31 years (range 4–89 years). Similar with previous studies, there was a slight female predominance with a M/F ratio of 1:1.3. The tumors involved the head and neck (n=16), arms (n=9), trunk and shoulders (n=9), foot (n=2), and hand (n=1). The clinical parameters are summarized in Table 1.
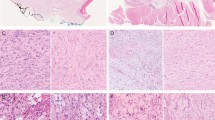
Histologically, all of the cases examined had at least focal areas of classic cellular neurothekeoma morphology with nests or bundles of epithelioid to spindled cells with oval nuclei and pinpoint nucleoli (Figures 1a and b). Although some cases had somewhat infiltrative edges, all were relatively well circumscribed. None had an extensively infiltrative or plexiform growth pattern. Twenty-three of the cases had areas with alternate growth patterns. In nine of the cases, the tumor had areas forming longer fascicles of spindled cells giving the tumor areas resembling cellular benign fibrous histiocytoma (Figure 1c). Further reminiscent of benign fibrous histiocytoma, nine cases had prominent collagen trapping (Figure 1d). Six of the cases demonstrated areas of little intervening collagen giving the tumor a sheet like growth pattern (Figure 2a). Four of the cases had at least some areas consistent with a desmoplastic cellular neurothekeoma with dense hyalinized collagen (Figure 2b). In the desmoplastic cellular neurothekeomas, some had a nested to cord-like arrangement of the tumor cells and myxohyaline stroma reminiscent of a myoepithelial tumor (Figure 2c). Two cases had small nests imparting a nevoid appearance at low power (Figure 2d).
(a) This cellular neurothekeoma had the classic nested growth pattern. (b) The tumor cells had an epithelioid appearance with relatively abundant cytoplasm with an open nuclear chromatin pattern and pinpoint nucleoli. (c) Fourteen cases had a prominent fascicular growth pattern. In this case, the tumor was reminiscent of a cellular benign fibrous histiocytoma. (d) In many cases, the tumor cells interdigitated around pre-existing collagen bundles of the reticular dermis (collagen trapping) similar to dermatofibromas.
(a) In six cases, there were areas with a confluent sheet-like growth pattern. (b) Four cases had features of desmoplastic cellular neurothekeoma with elongated nests and short fascicles embedded in a hyalinized collagenous stroma. (c) In this tumor, the tumor cells were arranged in cords and small nests with a myxohyaline chondroid stroma resembling a myoepithelial tumor. (d) Two cases had small nevus-like nests of tumor cells. (e) Myxoid stroma was present in many cases. In this case with abundant myxoid stroma, a swirling vaguely nested pattern is still evident. (f) In this case with abundant myxoid stroma, the tumor largely consisted of randomly arranged bland spindled cells.
Myxoid stroma was present in 14 cases. Of these cases, 11 had mildly increased myxoid stroma and 5 had prominent myxoid stroma. In most of the cases with myxoid stroma, the tumor cells still maintained a vaguely nested pattern reminiscent of cellular neurothekeoma (Figure 2e). In two cases with marked myxoid stroma, the tumor cells predominantly had a random arrangement to the individual tumor cells (Figure 2f). Focal nests of more conventional cellular neurothekeoma were, however, invariably present even in the most myxoid cases.
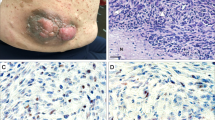
Perineural invasion/neurotropism was present in four cases (Figure 3a). The cases with perineural invasion were predominantly nested with two cases having focal fascicular growth. Two of the cases had a markedly myxoid stroma and one had mildly myxoid stroma. No case with perineural invasion had more than mild cytologic atypia, and they had low mitotic rates. None of the cases displayed vascular invasion.
(a) Perineural invasion was present in four cases. (b) Hemorrhage and osteoclast-like giant cells were present in two cases reminiscent of plexiform fibrohistiocytic tumor. Neither case had fibromatosis-like fascicles or a plexiform growth pattern. (c) In four cases, the tumor had vacuolated cytoplasm imparting a xanthomatous appearance to the tumor nests. (d) In one case, the tumor nests consisted of aggregates of eosinophilic cells with a scant lymphocytic cuff resembling sarcoidosis. (e) In another case, many of the tumor nests were surrounded by a relatively prominent lymphocytic cuff. (f) Moderate-to-severe cytologic atypia was present in 18 cases. In this case, pleomorphic and hyperchromatic atypical cells are admixed with more typical tumor cells of cellular neurothekeoma.
Multinucleated cells and osteoclastic giant cells were present in two cases. In both of these cases, there was evidence of hemorrhage or hemosiderin deposition (Figure 3b).
In four cases, there were areas where the tumor cells had vacuolated cytoplasm imparting a xanthomatous appearance to the tumor cells (Figure 3c). In one case, the tumor cells had a pseudo-granulomatous appearance somewhat reminiscent of sarcoidosis (Figure 3d). A cuff of lymphocytes was variably present around tumor nests in two cases (Figure 3e).
Cytologic atypia was mild in 19 cases with moderate and severe cytologic atypia being present in 15 and 3 cases, respectively, with tumor cells that had enlarged hyperchromatic nuclei. The atypical cells were admixed within a background of more conventional appearing tumor cells (Figure 3f).
The mitotic rate ranged from 0 to 10 mitotic figures per mm2 with a mean mitotic rate of 2/mm2. In general, the cases clustered into two groups those that had little to no mitotic figures and those with significant numbers of mitotic figures (≥5 mitotic figures/mm2; Figure 4a). Atypical mitotic figures were also identified in one case (Figure 4b). The case with atypical mitotic figures had moderate to focally severe cytologic atypia but also areas more resembling a conventual cellular neurothekeoma. Cases with atypia tended to have a higher mitotic rate than cases with only mild atypia, although there was overlap.
(a) Mitotic figures were identified in most cases. This image is from a typical cellular neurothekeoma. (b) One of the cases with prominent cytologic atypia had a rare atypical mitotic figure. (c) All of the cases were immunoreactive for NKI-C3. (d) With the exception of one case, all were positive for CD10.
Several cases had a combination of the above histologic features (Table 2). All of the tumors invariably had at least focal areas of the more typical nested pattern of cellular neurothekeoma.
All of the cases in which NKIC3 immunohistochemical stains were performed (n=34) were positive (Figure 4c), with only one case demonstrating weak immunoreactivity. CD10 was strongly positive in a 29 of 30 cases in which it was performed (Figure 4d). The one CD10-negative case had areas of classic morphologic features of cellular neurothekeoma. Positive immunoreactivity for MiTF was seen in 2/3 (67%) cases. Immunoreactivity for CD68 was seen in 6/13 (46%). Staining for smooth muscle actin was performed on 14 cases and was negative in all but 1 case. Immunohistochemical stains for S100 protein were negative in all 34 cases in which it was performed.
Discussion
Neurothekeomas were first described by Harkin and Reed in 1969 under the name nerve sheath myxoma.10 They were then reclassified under the name neurothekeoma by Gallager and Helwig in 1980.11 It was noted that lesions classified under this name had a varied histologic appearance, leading some to separate the lesions info myxoid, mixed, and cellular lesions.12 This was understandable given the morphologic overlap. Fetsch et al posited in 2005 that these were in fact two separate entities: dermal nerve sheath myxoma, a true nerve sheath tumor, and cellular neurothekeoma, a likely fibrohistocytic neoplasm.13 The myxoid variants differed from cellular neurothekeoma by their distinct septated growth pattern, prominent myxoid stroma and expression of S100 protein and GFAP. This distinction has been supported by other immunohistochemical and ultrastructural evidence.14 Cellular neurothekeoma consistently lacks expression of nerve sheath markers such as S100 protein2, 12 and ultrastructural examination has not demonstrated evidence of nerve sheath differentiation by electron microscopy.1, 15 Some authors have suggested that cellular neurothekeoma has nerve sheath differentiation by virtue of expression of PGP9.5,16 but this is completely nonspecific, as a wide range of tumors express this ubiquitin-related hydratase.17 The evidence that cellular neurothekeoma is most likely not a true nerve sheath tumor underlines the principle that morphologic overlap does not always equate with true relationship. In terms of nomenclature, we have come full circle. In the current WHO classification, dermal nerve sheath myxoma is now the preferred term to avoid confusion with cellular neurothekeoma.18
The true nature of cellular neurothekeoma remains elusive. There have been many theories regarding the histogenesis of these lesions with authors regarding them as variants of dermatofibromas, leiomyomas, or nerve sheath tumors.19, 20, 21, 22 It has been noted that cellular neurothekeoma can resemble a melanocytic tumor by virtue of its growth pattern, but lack of expression of S100 protein was against a melanocytic origin.12, 22 It is reasonable to classify cellular neurothekeoma as fibrohistiocytic tumors, as suggested by Fetsch et al,3 although this category is itself somewhat of a wastebasket term.
Regardless of the nosological debate, cellular neurothekeoma remains an entity fraught with diagnostic difficulty. Underscoring this difficulty is that in the majority of cases in this series, cellular neurothekeoma was not considered by the referring dermatopathologists/pathologists. This is not a reflection of diagnostic acumen but that cellular neurothekeoma has a greater degree of histologic plasticity than is widely appreciated.
Classically, cellular neurothekeoma is a usually relatively circumscribed, lobular tumor centered in the dermis. In some cases, the periphery of the tumor may have an infiltrative border. In lesions on the face this may result in the tumor infiltrating into underlying skeletal muscle. It is typically composed of nests of epithelioid to slightly spindled tumor cells that often have a subtle whorling pattern. The tumor cells have relatively abundant eosinophilic cytoplasm and round to oval nuclei with small pinpoint nucleoli.
By immunohistochemistry, cellular neurothekeoma is positive for the combination of NKI-C3 and CD10 in almost all cases.2, 3, 5, 23, 24 In our series, all of the cases tested were at least focally positive for NKI-C3 and 29/30 (97%) were positive for CD10. This parallels what has been previously reported in the literature. Immunoreactivity for these antibodies may be positive in a number of other lesions, so they must be interpreted in the correct histologic context.24 In our opinion, it is the combination of immunoreactivity for both of these markers is most helpful. Immunoreactivity for S100A6 has also been reported to have the same degree of sensitivity in cellular neurothekeoma.25, 26, 27, 28 Other antibodies are variably helpful. Immunoreactivity for MiTF has been seen in 81–100% of cellular neurothekeoma.23, 29 In this series, two of three cases tested were positive for MiTF. Immunostains for CD68 are insufficiently sensitive, as just under half of the cases tested were positive (6/13). Immunoreactivity for smooth muscle actin has been reported in up to 57% cases,2 but in our experience this is less common, being present in 1/14 cases in our series. PGP9.5 was once touted as a sensitive marker but lacks sufficient specificity to be useful.16, 17
Prominent myxoid stroma is a finding that is uncommon in cellular neurothekeoma, generally present in <10% of cases.2 This feature was somewhat more common in this series, present in five (14%) of our cases. In two of our cases, the myxoid stroma was so prominent that the nested growth pattern of cellular neurothekeoma was almost completely obscured, requiring very thorough examination in order not to miss the focus of more conventional appearing cellular neurothekeoma. The prominent myxoid stroma can cause confusion with dermal nerve sheath myxoma. Our cases with myxoid stroma lacked the fibrous septations and immunoreactivity for S100 protein characteristic of that entity.13, 18 Superficial angiomyxoma could also be considered, but this tumor lacks the nests of tumor cells seen in cellular neurothekeoma.30 In cases with myxoid stroma and atypia, a myxofibrosarcoma could be considered in the differential diagnosis, but this tumor is centered in the subcutis rather than dermis and has a very infiltrative growth pattern.18
The cases of cellular neurothekeoma with a fascicular growth pattern and peripheral collagen trapping were reminiscent of the cellular benign fibrous histiocytoma variant of dermatofibroma. This histologic variant of cellular neurothekeoma has been previously described.31 Similar to cellular neurothekeoma, cellular benign fibrous histiocytoma affects young adults, but with a slight male predominance.32, 33 In our opinion, the optimal method of distinguishing cellular neurothekeoma from cellular benign fibrous histiocytoma is to look for areas of classic cellular neurothekeoma. Although cellular neurothekeoma can occasionally exhibit fascicular growth, high mitotic rate, and collagen trapping, similar to cellular benign fibrous histiocytoma, the reverse is not true; cellular benign fibrous histiocytoma does not display areas of nested growth typical of cellular neurothekeoma. Although there are histologic differences, the two entities show molecular overlap with upregulation of the same genes involved in extracellular matrix growth and remodeling, including dermatopontin (DPT), a disintegrin and metalloproteinase domain 12 (ADAM12), matrix metalloproteinase 1 (MMP1), and periston osteoblast-specific factor (POSTN).34 This underscores the classification of cellular neurothekeoma as a so-called fibrohistiocytic tumor.
The cases that had tumor nodules with osteoclast-like giant cells and hemorrhage raise the differential diagnosis of plexiform fibrohistiocytic tumor. Indeed, these entities have significant histologic overlap, leading some authors to suggest that they may be the same entity.35 Although it is tempting to lump these tumors together based on morphologic similarity, we consider this equivalent to the erroneous classification of cellular neurothekeoma and dermal nerve sheath myxoma as the same entity based on histologic overlap. None of our cases had a plexiform growth pattern that is a defining characteristic of plexiform fibrohistiocytic tumor. The biologic behavior of the two entities is distinctly different. In stark contrast to cellular neurothekeoma, which almost never recurs and essentially never metastasizes (even cases with atypical features), plexiform fibrohistiocytic tumor has a relatively high rate of local recurrence and at least a low risk of metastasis.36, 37, 38 There are some immunophenotypic differences as well. Cellular neurothekeoma is also usually immunoreactive for MiTF and podoplanin, unlike plexiform fibrohistiocytic tumor.29, 39
Perineural invasion is a feature present in four of our cases. This has been previously highlighted as a histologic feature that is occasionally encountered in this entity.2, 40 This underscores the fact that perineural invasion may be seen in benign entities. The biologic significance of this finding is entirely dependent on the context of the neoplasm displaying this feature.
As expected mitotic activity was generally low in our series. However, 6/37 (16%) were mitotically active with ≥5 mitotic figures per mm2 (≥10 mitotic figures/10 HPFs). Similar high mitotic rates have been reported in 5–14% of cases2, 3 One case had atypical mitotic figures, but atypical mitotic figures have been noted in up to 9% of cases.3 Therefore, increased mitotic rates and atypical mitotic figures do not exclude cellular neurothekeoma from diagnostic consideration. Admittedly, a high mitotic rate or atypical mitotic figures should prompt careful scrutiny for typical features of cellular neurothekeoma before rendering the diagnosis.
Although cytologic atypia is not generally ascribed to cellular neurothekeoma, it is relatively common.2, 41, 42, 43 Significant cytologic atypia was seen in 9/37 (24%) cases with 1 case showing marked atypia and pleomorphism. This is in line with what has been previously reported, but cytologic atypia is still not widely recognized as a relatively common feature of cellular neurothekeoma.2
The presence of cytologic atypia or involvement of the distal extremities could prompt consideration of true sarcomas in the differential diagnosis. Epithelioid sarcoma has a nested growth pattern that can be similar to cellular neurothekeoma and, like cellular neurothekeoma, frequently presents in relatively young patients.18, 44, 45, 46 The distribution is different, as epithelioid sarcoma is distinctly more common in the distal extremities, although three of our cases presented on the distal extremities. The tumor nodules of epithelioid sarcoma often have necrosis, features not seen in our cases or other series of cellular neurothekeoma. Epithelioid sarcoma expresses cytokeratins, unlike cellular neurothekeoma, which readily allows for distinction.
In cases with marked pleomorphism, the diagnosis of atypical fibroxanthoma or a pleomorphic dermal sarcoma could be considered. These are less likely considerations as they are tumors that present on sun-damaged skin of the head and neck of elderly patients.47 Immunostains have little role in the distinction from cellular neurothekeoma, as atypical fibroxanthoma can be positive for NKI-C3 and CD10.48, 49
Finally, because of the nested growth pattern of cellular neurothekeoma, melanocytic tumors such as an intradermal Spitz nevus or metastatic melanoma could be considered in the differential diagnosis. It should be remembered that stains for NKI-C3 are positive in melanocytic tumors.50 Unlike true melanocytic tumors, cellular neurothekeoma is negative for S100 protein and more melanocyte-specific markers such as Melan-A and HMB-45.
In conclusion, we have summarized our experience with cellular neurothekeoma. Although most cases of cellular neurothekeoma are relatively straightforward to diagnose, we have encountered a number of cases, primarily in consultation, with unusual features. Cellular neurothekeoma is a tumor with a broader spectrum of histologic features than is appreciated. Recognition of at least focal areas of more conventional cellular neurothekeoma, especially the nested growth pattern, allows for accurate diagnosis of cellular neurothekeoma with atypical features. Immunohistochemical stains for NKI-C3 and CD10 are useful ancillary tests for the diagnosis of cellular neurothekeoma but they need to be interpreted in a strict histologic context. As previously discussed, cellular neurothekeoma, even tumors with atypical features, are benign. The presence of atypical features does not appear to impact behavior. Therefore, recognition of this entity is important to avoid the pitfall of mislabeling a cellular neurothekeomas as something more sinister.
References
Wartchow EP, Goin L, Schreiber J et al. Plexiform fibrohistiocytic tumor: ultrastructural studies may aid in discrimination from cellular neurothekeoma. Ultrastruct Pathol 2009;33:286–292.
Hornick JL, Fletcher CDM . Cellular neurothekeoma: detailed characterization in a series of 133 cases. Am J Surg Pathol 2007;31:329–340.
Bhatia S, Chu P, Weinberg JM . Atypical cellular neurothekeoma. Dermatol Surg 2003;29:1154–1157.
Fetsch JF, Laskin WB, Hallman JR et al. Neurothekeoma: an analysis of 178 tumors with detailed immunohistochemical data and long-term patient follow-up information. Am J Surg Pathol 2007;31:1103–1114.
Busam KJ, Mentzel T, Colpaert C et al. Atypical or worrisome features in cellular neurothekeoma: a study of 10 cases. Am J Surg Pathol 1998;22:1067–1072.
Campanati A, Brandozzi G, Sisti S et al. Atypical neurothekeoma: a new case and review of the literature. J Cutan Pathol 2007;34:435–437.
Mertz KD, Mentzel T, Grob M et al. A rare case of atypical cellular neurothekeoma in a 68-year-old woman. J Cutan Pathol 2009;36:1210–1214.
Wilson ADH, Rigby H, Orlando A . Atypical cellular neurothekeoma–a diagnosis to be aware of. J Plast Reconstr Aesthet Surg 2008;61:186–188.
Zedek DC, White WL, McCalmont TH . Desmoplastic cellular neurothekeoma: Clinicopathological analysis of twelve cases. J Cutan Pathol 2009;36:1185–1190.
Harkin JC, Reed RJ . Atlas of Tumor Pathology: Tumors of the Peripheral Nervous System Second series fascicle 3. Armed Forces Institute of Pathology: Washington, DC, 1969.
Gallager RL, Helwig EB . Neurothekeoma–a benign cutaneous tumor of neural origin. Am. J Clin Pathol 1980;74:759–764.
Husain S, Silvers DN, Halperin AJ et al. Histologic spectrum of neurothekeoma and the value of immunoperoxidase staining for S-100 protein in distinguishing it from melanoma. Am J Dermatopathol 1994;16:496–503.
Fetsch JF, Laskin WB, Miettinen M . Nerve sheath myxoma: a clinicopathologic and immunohistochemical analysis of 57 morphologically distinctive, S-100 protein- and GFAP-positive, myxoid peripheral nerve sheath tumors with a predilection for the extremities and a high local recurrence rate. Am J Surg Pathol 2005;29:1615–1624.
Argenyi ZB, Kutzner H, Seaba MM . Ultrastructural spectrum of cutaneous nerve sheath myxoma/cellular neurothekeoma. J Cutan Pathol 1995;22:137–145.
Pasquinelli G . Fibrohistiocytic tumors containing zebra body-like inclusions and fibripositors. Ultrastruct Pathol 2010;34:366–370.
Wang AR, May D, Bourne P et al. PGP9.5: a marker for cellular neurothekeoma. Am J Surg Pathol 1999;23:1401–1407.
Campbell LK, Thomas JR, Lamps LW et al. Protein gene product 9.5 (PGP 9.5) is not a specific marker of neural and nerve sheath tumors: an immunohistochemical study of 95 mesenchymal neoplasms. Mod Pathol 2003;16:963–969.
Fletcher CDM, Bridge JA, Hogendoorn PCW et al. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press: Lyon, 2013.
Calonje E, Wilson-Jones E, Smith NP et al. Cellular ‘neurothekeoma’: an epithelioid variant of pilar leiomyoma? Morphological and immunohistochemical analysis of a series. Histopathology 1992;20:397–404.
Zelger BG, Steiner H, Kutzner H et al. Cellular ‘neurothekeoma’: an epithelioid variant of dermatofibroma? Histopathology 1998;32:414–422.
Webb JN . The histogenesis of nerve sheath myxoma: report of a case with electron microscopy. J Pathol 1979;127:35–37.
Barnhill RL, Mihm MC Jr. . Cellular neurothekeoma. A distinctive variant of neurothekeoma mimicking nevomelanocytic tumors. Am J Surg Pathol 1990;14:113–120.
Page RN, King R, Mihm MC Jr et al. Microphthalmia transcription factor and NKI/C3 expression in cellular neurothekeoma. Mod Pathol 2004;17:230–234.
Sachdev R, Sundram UN . Frequent positive staining with NKI/C3 in normal and neoplastic tissues limits its usefulness in the diagnosis of cellular neurothekeoma. Am J Clin Pathol 2006;126:554–563.
Fullen DR, Lowe L, Su LD . Antibody to S100a6 protein is a sensitive immunohistochemical marker for neurothekeoma. J Cutan Pathol 2003;30:118–122.
Plaza JA, Torres-Cabala C, Evans H et al. Immunohistochemical expression of S100A6 in cellular neurothekeoma: clinicopathologic and immunohistochemical analysis of 31 cases. Am J Dermatopathol 2009;31:419–422.
García-Gutiérrez M, Toussaint-Caire S, González-Sánchez P et al. Multiple desmoplastic cellular neurothekeomas localized to the face of a 16-year-old boy. Am J Dermatopathol 2010;32:841–845.
Vered M, Fridman E, Carpenter WM et al. Classic neurothekeoma (nerve sheath myxoma) and cellular neurothekeoma of the oral mucosa: immunohistochemical profiles. J Oral Pathol Med 2011;40:174–180.
Fox MD, Billings SD, Gleason BC et al. Expression of MiTF may be helpful in differentiating cellular neurothekeoma from plexiform fibrohistiocytic tumor (histiocytoid predominant) in a partial biopsy specimen. Am J Dermatopathol 2012;34:157–160.
Calonje E, Guerin D, McCormick D et al. Superficial angiomyxoma:clinicopathologic analysis of a series of distinctive but poorly recognized cutaneous tumors with tendency for recurrence. Am J Surg Pathol 1999;23:910–917.
Thakral B, Gleason BC, Thomas AB et al. Cellular neurothekeoma with fascicular growth features mimicking cellular dermatofibroma. Am J Dermatopathol 2011;33:281–284.
Calonje E, Mentzel T, Fletcher CD . Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol 1994;18:668–676.
Franquemont DW, Cooper PH, Shmookler BM et al. Benign fibrous histiocytoma of the skin with potential for local recurrence: a tumor to be distinguished from dermatofibroma. Mod Pathol 1990;3:158–163.
Sheth S, Li X, Binder S et al. Differential gene expression profiles of neurothekeomas and nerve sheath myxomas by microarray analysis. Mod Pathol 2011;24:343–354.
Jaffer S, Ambrosini-Spaltro A, Mancini AM et al. Neurothekeoma and plexiform fibrohistiocytic tumor: mere histologic resemblance or histogenetic relationship? Am J Surg Pathol 2009;33:905–913.
Enzinger FM, Zhang RY . Plexiform fibrohistiocytic tumor presenting in children and young adults. An analysis of 65 cases. Am J Surg Pathol 1988;12:818–826.
Remstein ED, Arndt CA, Nascimento AG . Plexiform fibrohistiocytic tumor: clinicopathologic analysis of 22 cases. Am J Surg Pathol 1999;23:662–670.
Moosavi C, Jha P, Fanburg-Smith JC . An update on plexiform fibrohistiocytic tumor and addition of 66 new cases from the Armed Forces Institute of Pathology, in honor of Franz M. Enzinger, MD. Ann Diagn Pathol 2007;11:313–319.
Kaddu S, Leinweber B . Podoplanin expression in fibrous histiocytomas and cellular neurothekeomas. Am J Dermatopathol 2009;31:137–139.
Cardoso J, Calonje E . Cellular neurothekeoma with perineural extension: a potential diagnostic pitfall. J Cutan Pathol 2012;39:662–664.
Busam KJ, Mentzel T, Colpaert C et al. Atypical or worrisome features in cellular neurothekeoma: a study of 10 cases. Am J Surg Pathol 1998;22:1067–1072.
Mertz KD, Mentzel T, Grob M et al. A rare case of atypical cellular neurothekeoma in a 68-year-old woman. J Cutan Pathol 2009;36:1210–1214.
Gullett AE, Jimenez-Quintero LP, Prieto VG et al. Atypical cellular neurothekeoma in a pregnant woman. Arch Dermatol 2011;147:1342–1343.
Enzinger FM . Epitheloid sarcoma. A sarcoma simulating a granuloma or a carcinoma. Cancer 1970;26:1029–1041.
Halling AC, Wollan PC, Pritchard DJ et al. Epithelioid sarcoma: a clinicopathologic review of 55 cases. Mayo Clin Proc 1996;71:636–642.
Fisher C . Epithelioid sarcoma of Enzinger. Adv Anat Pathol 2006;13:114–121.
Miller K, Goodlad JR, Brenn T . Pleomorphic dermal sarcoma: adverse histologic features predict aggressive behavior and allow distinction from atypical fibroxanthoma. Am J Surg Pathol 2012;36:1317–1326.
Ma CK, Zarbo RJ, Gown AM . Immunohistochemical characterization of atypical fibroxanthoma and dermatofibrosarcoma protuberans. Am J Clin Pathol 1992;97:478–483.
Wieland CN, Dyck R, Weenig RH et al. The role of CD10 in distinguishing atypical fibroxanthoma from sarcomatoid (spindle cell) squamous cell carcinoma. J Cutan Pathol 2011;38:884–888.
Pernick NL, DaSilva M, Gangi MD et al. ‘Histiocytic markers’ in melanoma. Mod Pathol 1999;12:1072–1077.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Stratton, J., Billings, S. Cellular neurothekeoma: analysis of 37 cases emphasizing atypical histologic features. Mod Pathol 27, 701–710 (2014). https://doi.org/10.1038/modpathol.2013.190
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2013.190
Keywords
This article is cited by
-
An unusual case of neurothekeoma of the arm in an adult
Journal of Orthopaedics and Traumatology (2016)