Abstract
The current World Health Organization classification divides endometrial sarcomas into low-grade endometrial stromal sarcoma and undifferentiated endometrial sarcoma. Recent studies suggest undifferentiated endometrial sarcoma is a heterogeneous group and a subgroup with uniform nuclei is more akin to low-grade endometrial stromal sarcoma in terms of morphologic, immunohistochemical and genetic features. We classified endometrial sarcomas treated at our institution from 1998 to 2011 into low-grade endometrial stromal sarcoma and undifferentiated endometrial sarcoma, the latter being further categorized into a group with either uniform or pleomorphic nuclei. Morphological features, immunoprofile and fluorescence in situ hybridization rearrangements of JAZF1 and PHF1 genes were correlated with tumor category and outcome. A total of 40 cases were evaluated comprising 23 low-grade endometrial stromal sarcomas, 10 undifferentiated endometrial sarcomas with nuclear uniformity and 7 undifferentiated endometrial sarcomas with nuclear pleomorphism. Low-grade endometrial stromal sarcomas were more often estrogen and progesterone receptor positive (83%) compared with undifferentiated endometrial sarcoma with nuclear uniformity (10%) or with nuclear pleomorphism (0%) (P<0.001). Positivity for p53 was restricted to undifferentiated endometrial sarcomas with more frequent expression in the group with nuclear pleomorphism (57%) than with nuclear uniformity (10%) (P=0.06). Ki-67 proliferation index in >10% of tumor cells more frequent in undifferentiated endometrial sarcoma than low-grade endometrial stromal sarcoma (P=<0.001). JAZF1 rearrangement was detected in 32% of low-grade endometrial stromal sarcomas and in none of the undifferentiated sarcomas. Rearrangement of PHF1 was found in two patients, one with JAZF1−PHF1 fusion. There were no significant differences in clinical behavior between undifferentiated endometrial sarcoma with nuclear uniformity versus nuclear pleomorphism. In conclusion, we found undifferentiated endometrial sarcoma subtypes and low-grade endometrial stromal sarcoma have distinct immunohistochemical and cytogentic profiles. Our data do not show any difference in clinical behavior between subgroups in undifferentiated sarcomas.
Similar content being viewed by others
Main
Endometrial sarcomas are uncommon tumors of the uterus accounting for <10% of uterine mesenchymal neoplasms.1 Classification of these tumors has been a matter of challenge and controversy. They were traditionally divided into low- and high-grade based on mitotic count. Later it was proposed that as the high-grade tumors do not show specific differentiation or histological resemblance to endometrial stromal cells, they should be designated as undifferentiated endometrial or uterine sarcoma.2 While the current World Health Organization (WHO) classification divides these tumors into low-grade endometrial stromal sarcomas and undifferentiated endometrial sarcoma,2 three-tier and four-tier classifications have been previously used and newer concepts may alter the classification schema. Low-grade endometrial stromal sarcoma is defined by its resemblance to proliferative phase endometrial stroma. The distinct morphological features include uniform cells, prominent whorling of tumor cells around spiral-like arterioles and a tongue-like pattern of myometrial and/or lymphovascular invasion.1, 3 Occasionally, these tumors may show unusual variations including epithelial sex cord-like patterns, presence of endometrioid glands, smooth muscle metaplasia, fibro-myxoid change or adipose and skeletal muscle differentiation.4, 5, 6, 7 Low-grade endometrial stromal sarcoma is also characterized by chromosomal translocations in a subset of cases, most commonly t(7;17)(p15;q21) involving zinc-finger genes, JAZF1 and SUZ1.8, 9, 10, 11, 12, 13, 14
The WHO identifies undifferentiated endometrial sarcoma as displaying marked cellular atypia and lacking morphologic evidence of an endometrial stromal phenotype. Older grading systems, however, allowed for a category of endometrial sarcomas with greater atypia than low-grade endometrial stromal sarcoma, whereas retaining some resemblance to endometrial stroma. Such tumors were labeled as ‘high-grade endometrial stromal sarcoma’ by the Armed Forces Institute of Pathology1 or endometrial stromal sarcoma with grades 2–3 atypia (out of a three-point scale for nuclear atypia) by the largest histopathological analysis of endometrial sarcomas.15 Both grading systems used a three-tier system, distinguishing a higher grade endometrial stromal sarcoma from a truly undifferentiated sarcoma of the uterus. Recently, Kurihara et al16 also explored the heterogeneity of non-low-grade endometrial stromal sarcoma by dividing them into ‘undifferentiated endometrial sarcoma with nuclear uniformity’ and ‘undifferentiated endometrial sarcoma with nuclear pleomorphism’. They compared these two groups and interestingly their data suggested that undifferentiated endometrial sarcoma with nuclear uniformity shares some genetic and immunohistochemical characteristics with low-grade endometrial stromal sarcoma, whereas undifferentiated endometrial sarcoma with nuclear pleomorphism was distinctly different. Specifically, undifferentiated endometrial sarcoma with nuclear uniformity and low-grade endometrial stromal sarcoma showed increased estrogen receptor (ER) and progesterone receptor (PR) expression, JAZF1−SUZ1 rearrangement and absence of p53 mutation. In terms of biologic behavior, all low-grade endometrial stromal sarcomas showed a better overall survival compared with undifferentiated endometrial sarcoma with nuclear uniformity and undifferentiated endometrial sarcoma with nuclear pleomorphism both of which showed poor outcome.
Our objective was to study the clinical behavior, immunohistochemical profile and JAZF1−PHF1 rearrangement status of low-grade and non-low-grade endometrial stromal sarcoma. We also subdivided non-low-grade sarcomas into those with nuclear uniformity and nuclear pleomorphism as defined by Kurihara et al16 in order to further characterize the immunohistochemical and cytogenetic features of these two groups.
Materials and methods
Clinicopathologic Data
After obtaining approval by the research ethics board, the pathology database of University Health Network and cancer registry at Princess Margaret Hospital were searched for all cases of uterine sarcoma diagnosed as low-grade endometrial stromal sarcoma, high-grade endometrial stromal sarcoma and undifferentiated endometrial sarcoma between 1990 and 2011. Forty cases with tissue available for study were retrieved. Hematoxylin and eosin-stained slides were concurrently reviewed by two gynecologic pathologists. Morphological parameters were documented including: nuclear atypia (grades 1, 2 or 3), necrosis, mitotic index, atypical mitosis, pattern of myometrial invasion (infiltrating versus tongue-like invasion), similarity to proliferative phase endometrial stroma and arteriole-like vascular proliferation (Figure 1a). Differential diagnoses such as cellular leiomyoma, leiomyosarcoma, carcinosarcoma, adenosarcoma with stromal overgrowth and undifferentiated carcinoma were carefully excluded by morphological review and immunohistochemical staining with: cytokeratins (AE1/AE3, CK18 and CAM5.2), myogenic markers (a-smooth muscle actin, h-caldesmon and desmin) and epithelial membrane antigen. The cases were classified as low-grade endometrial stromal sarcoma and undifferentiated endometrial sarcoma based on WHO classification. Undifferentiated endometrial sarcoma cases were further divided into two categories based on a paper by Kurihara et al16: undifferentiated endometrial sarcoma with nuclear uniformity and undifferentiated endometrial sarcoma with nuclear pleomorphism. Undifferentiated endometrial sarcoma with nuclear uniformity featured a proliferation of uniform tumor cells with minimal nuclear pleomorphism, however, cytological atypia was too great for categorization as low-grade endometrial stromal sarcoma (Figure 1b). Cases designated as undifferentiated endometrial sarcoma with nuclear pleomorphism showed marked variation in nuclear size and shape, tumor giant cells, bizarre nuclei and prominent nucleoli (Figure 1c).
Clinical data were collected from electronic patients records, including: pathologic stage, surgical treatment, adjuvant therapies, disease recurrence and survival outcome. Stage at disease presentation was determined according to the AJCC 7th edition17 and by reviewing pathology reports of the primary tumor. The pathological stage was reported in all cases except for patients who received neoadjuvant therapy. In this setting, the clinical stage at presentation was used to infer the pre-neoadjuvant extent of disease.
Immunohistochemistry
A tissue microarray was made of 36 cases. Representative areas of tumor on the hematoxylin and eosin-stained slides were marked for incorporation into the microarray. The selected area was mapped and marked on the corresponding paraffin block. Two 0.6 mm cores of tissue from each case were drilled and embedded in the tissue microarray paraffin block. Five cases that were not incorporated into the tissue microarray were evaluated with the identical antibodies on a representative tumor block.
Immuonhistochemical staining for CD10 (Novocastra, clone: 56C6, dilution: 1/500), p53 (Leica, clone: D07, dilution: 1/1000), Ki-67 (Novus Biologicals, clone: Mib-1, dilution: 1/1000), ER (Neomarkers, clone: SP1, dilution: 1/400) and PR (Dako, clone: PgR636, dilution: 1/80) was performed. Immunohistochemical analysis was carried out using the standard streptavidin–biotin complex technique, after microwave antigen retrieval on 4-μm sections of formalin-fixed, paraffin wax-embedded tissue. Appropriate positive and negative controls were run in parallel. The immunostained slides were reviewed and scored by two gynecological pathologists. The intensity of staining and proportion of tumor cells positive at the highest intensity of staining were recorded. The proportion of cells staining was scored from 0 to 4 (0: none, 1: <10%, 2: 10–33%, 3: 34–66%, 4: >66%) and the intensity of staining from 0 to 3 (0: none, 1: weak, 2: intermediate, 3: strong). Proportion and intensity scores were added to obtain a combined score ranging from 0 to 7.
ER and PR were categorized as positive with a combined score of >3. As a result of the known focal staining of CD10 in endometrial sarcomas, a score of >2 was considered positive. Relatively diffuse and/or strong staining was required to determine p53 expression thus positivity corresponded to a score of ≥5. For Ki67 tumors demonstrating Ki67 expression in >10% of the tumor cells were compared with tumors showing less expression.
JAZF1 and PHF1 Analysis
Fluorescence in situ hybridization (FISH) was employed to examine for rearrangement involving JAZF1 and/or PHF1, using flanking probe sets extracted from bacterial artificial chromosomes. RP11-597H8 and RP11-78F4 were used to generate the probes telomeric to JAZF1 and RP11-466B23 and RP11-945M23 for probes centromeric to JAZF1. RP11-242N19 and RP11-908F2 were used to generate the probes telomeric to PHF1 and RP11-94D23 for probe centromeric to PHF1. DNA extracted from bacterial artificial chromosome clones was directly labeled with Spectrum Green (RP11-597H8, RP11-78F4 and RP11-94D23) or Spectrum Orange (RP11-466B23, RP11-945M23, RP11-242N19 and RP11-908F2) by nick translation method (Vysis, Downer's Grove, IL, USA) with the labeled probes subsequently purified to remove excess label using Centri-Sep purification columns (Princeton Separations, Adelphia, NJ, USA) according to the manufacturer's protocols. The DNA was then precipitated, centrifuged and dried according to standard protocols. Each purified probe was resuspended in LSI Hybridization buffer (Vysis) to give 10 ng probe/μl with a 10-fold excess of Cot-1 DNA and 20-fold excess of human placental DNA. After confirming correct localization of the probe signals on metaphase preparation of normal human lymphocytes, probes were hybridized on the microarray slides using a protocol optimized for formalin-fixed paraffin-embedded tissue microarray section.18, 19
The hybridized tissue microarray slides were examined using a conventional fluorescence microscope. A total of 100 nuclei per core were evaluated. Normal paired signals are defined as an orange and green signal <3 signal diameters apart or a single yellow (overlapping) signal, whereas unpaired signals are those separated by ≥3 signal diameters from an oppositely colored signal. Only cases with clearly visible probe signals in at least 100 nuclei are considered interpretable and cases with weak signals were considered as non-interpretable. The following cutoff points were used: cases with abnormal signals detected in ≥30% of the tumor nuclei were considered as positive for rearrangement; cases with abnormal signals in <10% of nuclei were considered as negative. Cases with abnormal signals in 10–30% of the nuclei were considered as equivocal; there were no equivocal cases encountered in the current series.
Statistical Analysis
Comparisons of categorical variables were done using the Fisher’s exact test.
Results
Clinicopathologic Features
Clinicopathologic findings of all cases are summarized in Table 1. The study cohort comprised 40 cases including 23 low-grade endometrial stromal sarcomas and 17 undifferentiated endometrial sarcomas. Low-grade endometrial stromal sarcomas consisted of 17 primary tumors (15 uterine and 2 ovarian primaries) and 6 recurrent tumors. Mean age of patients with low-grade endometrial stromal sarcoma was 50 years (range, 27–84). Undifferentiated endometrial sarcoma cases consisted of 10 with nuclear uniformity and 7 with nuclear pleomorphism. Most undifferentiated endometrial sarcomas were primary uterine tumors except for one pelvic mass and one lung metastases (cases 24 and 26). Mean age of patients was 61 years in the nuclear uniformity group and 60 years in the nuclear pleomorphism group (range, 47–75). Undifferentiated endometrial sarcoma patients were significantly older than those with low-grade endometrial stromal sarcomas (P<0.01).
Stage at presentation of disease for uterine low-grade endometrial stromal sarcoma was as follows: 14 at stage I, 3 at stage II, 2 at stage III and 1 at stage IV. In one case, the stage was unknown. Of cases presenting as stage I (confined to the uterus), six had cervical involvement. Cases 12 and 19 were both ovarian primaries and therefore not assigned a stage. The specimen analyzed in case 19 was a recurrent peritoneal low-grade endometrial stromal sarcoma, whereas case 12 was a primary ovarian low-grade endometrial stromal sarcoma. Both these cases were thought to arise from ovarian endometriotic foci although histopathological evidence of endometriosis was found in case 12 only. Among undifferentiated endometrial sarcomas with nuclear uniformity, five cases presented at stage I, one at stage II, three at stage III and one of unknown stage. Five undifferentiated endometrial sarcoma with nuclear pleomorphism presented at stage I and two at stage II. There was no difference in stage at presentation between low-grade endometrial stromal sarcomas and the undifferentiated subtypes.
Low-grade endometrial stromal sarcoma patients experienced local or distant recurrence of their disease an average of 46 months after the initial diagnosis. Eleven patients had adjuvant radiotherapy and one patient (case 4) received neoadjuvant radiation. Six patients had adjuvant hormonal therapy with agents that included: medroxyprogesterone, estradiol, megestrol or leuprolide. Two patients received chemotherapy: case 15 had neoadjuvant treatment with paclitaxel/carboplatin and case 18 had doxorubicin/cisplatin. One patient (case 18) died of her disease 132 months from her initial diagnosis with local and distant metastases. All others were alive at the time of most recent follow-up.
Distant or local recurrence occurred after an average of 16.7 months in patients with undifferentiated endometrial sarcoma with nuclear uniformity and 6.2 months in those with nuclear pleomorphism. In all, 12 patients with undifferentiated endometrial sarcoma had adjuvant radiotherapy (8 with nuclear uniformity and 4 with nuclear pleomorphism) and 11 had adjuvant chemotherapy (5 with nuclear uniformity and 6 with nuclear pleomorphism). Three patients with undifferentiated endometrial sarcoma died of their disease (two with nuclear uniformity and one with nuclear pleomorphism). One patient with undifferentiated endometrial sarcoma with nuclear pleomorphism (case 39) was not fit for surgery and died shortly after transfer to palliative unit. Another patient with undifferentiated endometrial sarcoma with nuclear uniformity as well as a history of colon cancer and lymphoma died 16 months after her sarcoma diagnosis, however, there was no evidence of progression of her uterine sarcoma at the time of her death (case 31).
In an analysis of clinical data, the presence of distant metastases and patients living with disease were more common in the undifferentiated endometrial sarcoma groups (Table 2).
Histopathologic Findings
In all, 22 of 23 low-grade endometrial stromal sarcomas demonstrated the classic appearance of uniform tumor cells with oval to spindle nuclei and whorling around small arteriole-like vessels. The one case lacking this appearance had prominent smooth muscle differentiation (case 8) with CD10 positivity in the majority of cells. Of the 17 cases of primary low-grade endometrial stromal sarcoma, 12 showed permeative ‘tongue-like’ myometrial infiltration, 11 had lymphovascular space invasion and 4 cases contained necrotic foci. Mitotic figures were variable with average 5.8 per 10 high-power fields (range, 0–20). Abnormal mitotic figures were not identified. We identified one case with fibromyxoid features (case 10), four with focal smooth muscle differentiation (cases 3, 8, 17 and 19) and three with focal sex cord-like differentiation (cases 2, 6 and 18). In cases with smooth muscle differentiation, diffuse staining with smooth muscle markers were not present.
All cases of undifferentiated endometrial sarcoma appeared as sheets of neoplastic cells with significant nuclear atypia without any specific differentiation. Bone-forming, heterologous elements were present in case 28. Whorling of tumor cells around arteriole-like vessels was not seen in any cases of undifferentiated endometrial sarcoma. Lymphovascular permeation was observed in 4 of 9 cases of primary undifferentiated endometrial sarcoma with nuclear uniformity and 4 of 7 cases with nuclear pleomorphism. Necrosis was identified in 8 of 9 cases with nuclear uniformity and 6 of 7 cases with nuclear pleomorphism. The average mitotic count was 38.3 per 10 high-power fields (range, 18–80) in undifferentiated endometrial sarcoma with nuclear uniformity and 57.8 per 10 high-power fields (range, 40–100) with nuclear pleomorphism. The frequency of necrosis and mitotic rates were both significantly higher in undifferentiated endometrial sarcomas compared with low-grade endometrial stroma sarcomas (P<0.001). Abnormal mitotic figures were seen in both types of undifferentiated endometrial sarcomas.
Immunohistochemistry Findings
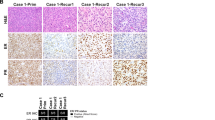
Immunohistochemical findings are summarized in Table 3. ER was positive in 83% of low-grade endometrial stromal sarcomas, 10% of undifferentiated endometrial sarcomas with nuclear uniformity and none of undifferentiated endometrial sarcomas with nuclear pleomorphism (P<0.001). The expression of PR correlated with ER in all cases. Positivity with p53 was restricted to undifferentiated endometrial sarcomas with more frequent expression in cases with nuclear pleomorphism than nuclear uniformity (57% versus 10%, P=0.06). Proliferation index with Ki-67 was also higher in undifferentiated endometrial sarcomas than low-grade endometrial stromal sarcomas (P<0.001). There were no other significant differences in immunohistochemical expression between the undifferentiated endometrial sarcoma subtypes (Figures 2 and 3).
Molecular Findings
FISH analysis for JAZF1 rearrangement analysis was interpretable in 35 cases (Table 3). The rearrangement was detected in 32% of low-grade endometrial stromal sarcomas and in none of the undifferentiated endometrial sarcomas (P=0.003). Rearrangement for PHF1 was seen in two low-grade endometrial stromal sarcomas: cases 2 and 15. Case 2 was also positive for JAZF1 rearrangement implying a JAZF1−PHF1 genetic fusion.
In case 18, the primary tumor was unavailable for study. Instead, three separate recurrences from the same patient were analyzed in the tissue microarray block. The patient’s first documented recurrence 7 years after initial diagnosis was in the bladder and was negative for JAZF1. A pelvic mass at 8 years was also negative, however, a soft tissue recurrence of her low-grade endometrial stromal sarcoma at 10 years was positive for JAZF1 mutation (this result is not included in Table 3). This was the only patient with low-grade endometrial stromal sarcoma to have died of her disease.
Discussion
Despite the variances in grading systems over time, the clinical behavior of low-grade and non-low-grade endometrial stromal sarcomas are relatively distinct. Low-grade endometrial stroma sarcoma is an indolent tumor prone to local recurrence, which affects up to one-half of patients.2, 20 Overall survival ranges from 65 to 76% at 10 years.21, 22 In contrast, undifferentiated endometrial sarcoma has an aggressive clinical course with death occurring usually within 3–5 years.2, 22 Our series was in agreement with these trends. Low-grade endometrial stromal sarcoma patients experienced local or distant recurrence an average of 46 months after the initial diagnosis compared with 16.7 months in undifferentiated endometrial sarcoma with nuclear uniformity and 6.2 months in undifferentiated endometrial sarcoma with nuclear pleomorphism patients. One of 23 low-grade endometrial stromal sarcoma patients with clinical follow-up died of her disease at 11 years compared with 2 of 8 patients with undifferentiated endometrial sarcoma with nuclear uniformity and 1 of 7 patients with nuclear pleomorphism. The undifferentiated endometrial sarcoma patients died between 3 weeks to 27 months after diagnosis. Although only one undifferentiated endometrial sarcoma with nuclear pleomorphism patient was confirmed as deceased, five others had advanced stages of disease and were either lost to follow-up or transferred to outside palliative care centers. Thus, the number of deaths in this group is likely underestimated.
In a comprehensive series of uterine sarcomas that included five undifferentiated endometrial sarcomas, three patients with tumors showing nuclear pleomorphism died within 1 year. The other two undifferentiated endometrial sarcomas with nuclear uniformity were alive at 2 and 9 years post surgical treatment. All three cases with pleomorphism presented with stage I disease.23 In Kurihara’s series,16 although all low-grade endometrial stromal sarcomas with clinical follow-up were alive, 4 of 7 patients with undifferentiated endometrial sarcomas with nuclear uniformity and 3 of 6 with nuclear pleomorphism died of their disease. Notably, all patients who were deceased presented at advanced stage (stage III or IV). It is difficult to compare outcomes in endometrial sarcomas across retrospective studies. First, they are infrequent neoplasms and most studies report small sample sizes. Second, the mode of treatment is variable among patients, especially with respect to adjuvant therapy. Thus, homogenous groups of patients with adequate follow-up are not available for comparison. However, our results were similar to those of Kurihara et al16 in that there appeared to be no difference in outcomes between undifferentiated endometrial sarcoma with nuclear uniformity and undifferentiated endometrial sarcoma with nuclear pleomorphism.
Several studies have reported older patient age in non-low-grade endometrial stroma sarcoma patients.1, 12, 16, 22 The age range for presentation of low-grade endometrial stromal sarcomas is 42–58 years.1 In contrast, mean patient age for non-low-grade tumors is 55–60 years.1, 22 Accordingly in this study, mean age of patients was significantly younger in the low-grade endometrial stromal sarcoma group (50 years) than undifferentiated endometrial sarcoma patients (61 years for undifferentiated endometrial sarcoma with nuclear uniformity and 60 years for those with nuclear pleomorphism). The age of our patients with undifferentiated endometrial sarcoma with nuclear uniformity is higher than that reported by Kurihara et al16 in which mean age was 41.5 years for undifferentiated endometrial sarcoma with nuclear uniformity and 59.5 years for those with nuclear pleomorphism.16 In terms of prognostic significance, a study of 831 endometrial sarcomas from the Surveillance Epidemiology and End Results database reported worse survival in women over 52 years.24
By far the most reliable prognostic factor in uterine sarcomas is stage.15, 20, 22, 24 However, the majority of uterine sarcomas in general present at stage I,16, 22, 24, 25, 26 therefore predicting behavior in low-stage tumors, particular low-grade endometrial stromal sarcoma, remains a challenge. The proportion of stage I tumors in our series was similarly high, constituting 61% of low-grade endometrial stromal sarcomas, 50% of undifferentiated endometrial sarcomas with nuclear uniformity and 29% of the nuclear pleomorphism group. Of patients who died of their disease all were stage I except for one undifferentiated endometrial sarcoma with nuclear pleomorphism patient presenting at stage II. Although stage did not appear to predict mortality in our series, the length of follow-up for several undifferentiated endometrial sarcoma cases was <1 year. This short follow-up may mitigate the outcome in some undifferentiated endometrial sarcoma cases.
Histological predictors of outcome in endometrial stromal tumors have also been previously sought. Mitotic activity and tumor necrosis have variably been associated with prognosis. In a study of 83 endometrial stromal sarcomas from the Norwegian Cancer Registry, both factors were related to poor outcome in tumors confined to the uterus.22 However, in the largest histopathologic series of endometrial stromal sarcomas to date, mitotic activity was not related to survival in stage I tumors15 and this finding has been corroborated by others.1, 27 Although necrosis and robust mitotic activity are not definitional of undifferentiated endometrial sarcoma, they are more commonly associated with these tumors in comparison with low-grade endometrial stromal sarcomas1, 2, 22, 27 and our results were no different. We did find that the average mitotic rate was higher in undifferentiated endometrial sarcoma with nuclear pleomorphism than in tumors with nuclear uniformity (58 per 10 high-power fields versus 38 per 10 high-power fields) but the result was not significant and neither type of undifferentiated endometrial sarcoma had <10 mitoses per 10 high-power fields. The frequency of necrosis between undifferentiated endometrial sarcoma with nuclear uniformity and with nuclear pleomorphism also did not differ. Atypical mitoses were seen in 5 of 9 primary undifferentiated endometrial sarcomas with nuclear uniformity and 5 of 7 with nuclear pleomorphism. This is in contrast to Kurihara’s series16 in which atypical mitoses were not seen in undifferentiated endometrial sarcomas with nuclear uniformity, another similarity with low-grade endometrial stromal sarcoma.
Numerous studies have explored the immunohistochemical phenotype of endometrial stromal sarcomas. Immunoreactivity for CD10, ER, PR, desmin, vimentin, smooth muscle actin, muscle-specific actin and beta-catenin have all been described.5, 28, 29, 30, 31, 32, 33, 34, 35 Of these, CD10 is the most sensitive for endometrial differentiation and rarely endometrial stromal sarcomas are negative for this marker.7, 23, 30 However, CD10 may be expressed in other uterine mesenchymal tumors such as leiomyosarcoma, cellular leiomyomas, rhabdomyosarcomas and carcinosarcomas5, 7, 30 as well as extra-uterine tumors like hemangiopericytoma and solitary fibrous tumor.29 In distinguishing endometrial stromal sarcoma from uterine smooth muscle neoplasms, H-caldesmon has been touted as a more specific marker whereas desmin, smooth muscle actin, muscle-specific actin and calponin have all been variably shown to stain endometrial sarcomas.7, 10, 30, 31
There are few studies in the literature addressing immunohistochemical findings of undifferentiated endometrial sarcoma in relation to low-grade tumors. Interestingly, our results showed CD10 expression in undifferentiated endometrial sarcoma as well as low-grade endometrial stromal sarcoma. Undifferentiated endometrial sarcoma and low-grade endometrial stromal sarcomas were better separated by ER, PR, Ki-67 and p53. Low-grade endometrial stromal sarcoma was more often positive for ER and PR, whereas a higher percentage of undifferentiated endometrial sarcoma (both subtypes) expressed proliferation index >10% in comparison with low-grade endometrial stromal sarcoma. p53 expression was restricted to undifferentiated endometrial sarcoma with more frequent expression in the pleomorphic than uniform group (57% versus 10%). Compared with our results, Kurihara et al16 reported greater ER expression in their undifferentiated endometrial sarcomas with nuclear uniformity group with 4 of 7 (57%) tumors staining. As well, both immunohistochemical expression and mutation analysis of p53 was seen in 3 of 6 cases of the undifferentiated endometrial sarcomas with nuclear pleomorphism group only. The immunohistochemical profile of ER and PR expression and negative p53 was significant in the combined group of low-grade endometrial stromal sarcoma and undifferentiated endometrial sarcoma with nuclear uniformity group compared with undifferentiated endometrial sarcoma with nuclear pleomorphism. Variations in immunohistochemical results between our study and Kurihara et al16 could be explained by different scoring systems in the evaluation of markers and/or different antibody clones for ER and p53. There is no specific immunohistochemistry marker to differentiate between these categories. The authors believe that the main diagnostic criteria to distinguish low-grade and non-low-grade tumors still remain to be based on histomorphological features defined by WHO: ‘undifferentiated endometrial sarcomas display marked cellular atypia and lack morphologic evidence of an endometrial stromal phenotype’. Although we believe that immunohistochemistry staining can assist the pathologist in certain cases, our results do not suggest certain selection criteria for performing immunohistochemistry studies. Nevertheless, the results of such ancillary studies should be interpreted carefully and along with morphological findings.
Currently, the non-low-grade endometrial sarcomas are managed similarly in many cancer centers therefore differentiating undifferentiated endometrial sarcoma with nuclear uniformity from undifferentiated endometrial sarcoma with nuclear pleomorphism does not seem to have clinical implication at the moment, however, this distinction and further molecular characterization might provide guidance toward more specific treatment options including possible targeted therapy in the future.
The fusion of the two zinc-finger genes JAZF1 and SUZ1 at the 7p15 and 17q21 breakpoints, respectively, was described in endometrial stromal tumors by Koontz et al in 2001.8 The function of this fusion transcript is still unknown. The prevalence of the associated translocation, t(7;17)(p15;q21) in low-grade endometrial stromal sarcoma by RT-PCR10, 13, 14, 16 and FISH11, 12 ranges from 23 to 80%. Our results are within this range with a 32% positivity rate. Although the JAZF1−SUZ1 rearrangement is the most common translocation in endometrial stromal tumors, more recently, rearrangements with the PHF1 (6p21) zinc-finger gene have been described in 1% of low-grade endometrial stromal sarcomas.9, 12 PHF1 was detected in two of our low-grade endometrial stromal sarcoma patients; one showed JAZF1−PHF1 fusion.
The number of undifferentiated endometrial sarcomas evaluated for t(7;17)(p15;q21) in the literature is low and so far only 2 of 17 (11.8%) were found to be positive.8, 12, 13, 16 In our series, we confirm that JAZF1 rearrangement is a rare occurrence in undifferentiated endometrial sarcomas with a total of 13 analyzed cases found to be negative. In Kurihara’s series,16 1 of 3 and 0 of 3 of undifferentiated endometrial sarcomas with nuclear uniformity and nuclear pleomorphism, harbored the JAZF1−SUZ1 transcript, respectively. They suggested that JAZF1−SUZ1 rearrangement may be a further commonality shared by undifferentiated endometrial sarcoma with nuclear uniformity and low-grade endometrial stromal sarcoma. Notably, the undifferentiated endometrial sarcoma with nuclear uniformity, which was positive for the rearrangement had focal areas morphologically resembling low-grade endometrial stromal sarcoma.
It has been postulated that the low frequency of the translocation in undifferentiated endometrial sarcoma is evidence that its pathway of tumorigenesis is separate from low-grade endometrial stromal sarcoma.8, 12, 16 However, there are a few cases in the literature reporting a direct transition from low-grade endometrial stromal sarcoma to a tumor resembling undifferentiated endometrial sarcoma.36, 37, 38 In two such cases, the recurrences of primary uterine low-grade endometrial stromal sarcoma demonstrated greater cellular atypia, loss of spiral arterioles and increased mitotic rate. Both patients died of their disease after 8 and 25 years.36 Cheung et al37 report the coexistence of a predominantly low-grade endometrial stromal sarcoma with an area of dedifferentiated ‘high-grade’ sarcoma in the same primary uterine tumor. The dedifferentiated area displayed large, pleomorphic nuclei, lack of staining with ER and PR and greater anueploidy. The patient was well 18 months later.
Conclusion
Undifferentiated endometrial sarcoma and low-grade endometrial stromal sarcoma have distinct immunohistochemical and cytogentic profiles. We were able to morphologically distinguish a subset of undifferentiated endometrial sarcoma with uniform nuclei, first described by Kurihara et al,16 from the pleomorphic, subtype.16 The undifferentiated endometrial sarcomas with nuclear uniformity and nuclear pleomorphism groups showed some differences in immunohistochemical profile. p53 expression is significantly more common in undifferentiated endometrial sarcomas with nuclear pleomorphism compared with those with nuclear uniformity and not found in low-grade endometrial stromal sarcoma. ER and PR expression is uncommon in undifferentiated endometrial sarcomas and when present, is only seen in the subtype with uniform nuclei. Although there appears to be some difference in tumorigenesis with more frequent p53 abnormalities seen in undifferentiated endometrial sarcomas with nuclear pleomorphism compared with undifferentiated endometrial sarcomas with nuclear uniformity, both appear to share a similarly aggressive course. Our data did not show any difference in behavior between the two categories of undifferentiated endometrial sarcoma, however, the morphological features and the immunoprofile suggest that that the non-low-grade tumors are a heterogeneous group of tumors. The authors believe that the older grading systems, which allowed for a category of endometrial sarcomas as high-grade endometrial stromal sarcoma might be still valid and this category might be equal to undifferentiated endometrial sarcoma with nuclear uniformity in our series. Larger studies with longer follow-up periods and further molecular investigations are needed to clarify this concept.
References
Silverberg SG, Kurman RJ . Endometrial stromal tumors In: Silverberg SG, Kurman RJ (eds) Atlas of Tumor Pathology: Tumors of the Uterine Corpus and Gestational Trophoblastic Disease. Third series, Fascicle 3. Armed Forces Institute of Pathology: Washington, 1992, pp 91–110.
Hendrickson MR, Tavassoli FA, Kempson RL, et al. Mesenchymal tumours and related lesions In: Tavassoli FA, Devilee P (eds) World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press: Lyon, 2003, pp 233–236.
Zaloudek CJ, Hendrickson MR, Soslow RA . Mesenchymal tumors of the uterus. In: Kurman RJ, Ellenson LH, Ronnett BM (eds) Blaustein’s Pathology of the Female Genital Tract, 6th edn. Springer: New York, 2011, pp 484–493.
Clement PB, Scully RE . Endometrial stromal sarcomas of the uterus with extensive endometrioid glandular differentiation: a report of three cases that caused problems in differential diagnosis. Int J Gynecol Pathol 1992;11:163–173.
Baker P, Oliva E . Endometrial stromal tumours of the uterus: a practical approach using conventional morphology and ancillary techniques. J Clin Pathol 2007;60:235–243.
Yilmaz A, Rush DS, Soslow RA . Endometrial stromal sarcomas with unusual histologic features: a report of 24 primary and metastatic tumors emphasizing fibroblastic and smooth muscle differentiation. Am J Surg Pathol 2002;26:1142–1150.
Oliva E, Young RH, Clement PB, et al. Myxoid and fibrous endometrial stromal tumors of the uterus: a report of 10 cases. Int J Gynecol Pathol 1999;18:310–319.
Koontz JI, Soreng AL, Nucci M, et al. Frequent fusion of the JAZF1 and JJAZ1 genes in endometrial stromal tumors. Proc Natl Acad Sci USA 2001;98:6348–6353.
Micci F, Walter CU, Teixeira MR, et al. Cytogenetic and molecular genetic analyses of endometrial stromal sarcoma: nonrandom involvement of chromosome arms 6p and 7p and confirmation of JAZF1/JJAZ1 gene fusion in t(7;17). Cancer Genet Cytogenet 2003;144:119–124.
Nucci MR, Harburger D, Koontz J, et al. Molecular analysis of the JAZF1-JJAZ1 gene fusion by RT-PCR and fluorescence in situ hybridization in endometrial stromal neoplasms. Am J Surg Pathol 2007;31:65–70.
Oliva E, de Leval L, Soslow RA, et al. High frequency of JAZF1-JJAZ1 gene fusion in endometrial stromal tumors with smooth muscle differentiation by interphase FISH detection. Am J Surg Pathol 2007;31:1277–1284.
Chiang S, Ali R, Melnyk N, et al. Frequency of known gene rearrangements in endometrial stromal tumors. Am J Surg Pathol 2011;35:1364–1372.
Hrzenjak A, Moinfar F, Tavassoli FA, et al. JAZF1/JJAZ1 gene fusion in endometrial stromal sarcomas: molecular analysis by reverse transcriptase-polymerase chain reaction optimized for paraffin-embedded tissue. J Mol Diagn 2005;7:388–395.
Huang HY, Ladanyi M, Soslow RA . Molecular detection of JAZF1-JJAZ1 gene fusion in endometrial stromal neoplasms with classic and variant histology: evidence for genetic heterogeneity. Am J Surg Pathol 2004;28:224–232.
Chang KL, Crabtree GS, Lim-Tan SK, et al. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol 1990;14:415–438.
Kurihara S, Oda Y, Ohishi Y, et al. Endometrial stromal sarcomas and related high-grade sarcomas: immunohistochemical and molecular genetic study of 31 cases. Am J Surg Pathol 2008;32:1228–1238.
Edge SB, Byrd DR, Compton CC, et al. Corpus uteri In: Edge SB, Byrd DR, Compton CC, et al. (eds) American Joint Committee on Cancer., American Cancer Society. AJCC cancer staging handbook: from the AJCC cancer staging manual, 7th edn. Springer: New York, 2010, pp 487–488.
Lee CH, Huntsman DG, Cheang MC, et al. Assessment of Her-1, Her-2, And Her-3 expression and Her-2 amplification in advanced stage ovarian carcinoma. Int J Gynecol Pathol 2005;24:147–152.
Terry J, Barry TS, Horsman DE, et al. Fluorescence in situ hybridization for the detection of t(X;18)(p11.2;q11.2) in a synovial sarcoma tissue microarray using a breakapart-style probe. Diagn Mol Pathol 2005;14:77–82.
Chew I, Oliva E . Endometrial stromal sarcomas: a review of potential prognostic factors. Adv Anat Pathol 2010;17:113–121.
Norris HJ, Taylor HB . Mesenchymal tumors of the uterus. I. A clinical and pathological study of 53 endometrial stromal tumors. Cancer 1966;19:755–766.
Abeler VM, Royne O, Thoresen S, et al. Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology 2009;54:355–364.
D’Angelo E, Prat J . Uterine sarcomas: a review. Gynecol Oncol 2010;116:131–139.
Chan JK, Kawar NM, Shin JY, et al. Endometrial stromal sarcoma: a population-based analysis. Br J Cancer 2008;99:1210–1215.
D’Angelo E, Spagnoli LG, Prat J . Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the World Health Organization classification system. Hum Pathol 2009;40:1571–1585.
Al Wakiel H, Ragheb AM, Varghese A, et al. Uterine sarcoma: 14 years experience in KCCC. Gulf J Oncolog 2008;45–51.
Evans HL . Endometrial stromal sarcoma and poorly differentiated endometrial sarcoma. Cancer 1982;50:2170–2182.
Balleine RL, Earls PJ, Webster LR, et al. Expression of progesterone receptor A and B isoforms in low-grade endometrial stromal sarcoma. Int J Gynecol Pathol 2004;23:138–144.
Bhargava R, Shia J, Hummer AJ, et al. Distinction of endometrial stromal sarcomas from 'hemangiopericytomatous' tumors using a panel of immunohistochemical stains. Mod Pathol 2005;18:40–47.
Chu PG, Arber DA, Weiss LM, et al. Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: an immunohistochemical comparison of 34 cases. Mod Pathol 2001;14:465–471.
Farhood AI, Abrams J . Immunohistochemistry of endometrial stromal sarcoma. Hum Pathol 1991;22:224–230.
Franquemont DW, Frierson HF, Mills SE . An immunohistochemical study of normal endometrial stroma and endometrial stromal neoplasms. Evidence for smooth muscle differentiation. Am J Surg Pathol 1991;15:861–870.
Jung CK, Jung JH, Lee A, et al. Diagnostic use of nuclear beta-catenin expression for the assessment of endometrial stromal tumors. Mod Pathol 2008;21:756–763.
Kurihara S, Oda Y, Ohishi Y, et al. Coincident expression of beta-catenin and cyclin D1 in endometrial stromal tumors and related high-grade sarcomas. Mod Pathol 2010;23:225–234.
Reich O, Regauer S, Urdl W, et al. Expression of oestrogen and progesterone receptors in low-grade endometrial stromal sarcomas. Br J Cancer 2000;82:1030–1034.
Amant F, Woestenborghs H, Vandenbroucke V, et al. Transition of endometrial stromal sarcoma into high-grade sarcoma. Gynecol Oncol 2006;103:1137–1140.
Cheung AN, Ng WF, Chung LP, et al. Mixed low grade and high grade endometrial stromal sarcoma of uterus: differences on immunohistochemistry and chromosome in situ hybridisation. J Clin Pathol 1996;49:604–607.
Ohta Y, Suzuki T, Omatsu M, et al. Transition from low-grade endometrial stromal sarcoma to high-grade endometrial stromal sarcoma. Int J Gynecol Pathol 2010;29:374–377.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jakate, K., Azimi, F., Ali, R. et al. Endometrial sarcomas: an immunohistochemical and JAZF1 re-arrangement study in low-grade and undifferentiated tumors. Mod Pathol 26, 95–105 (2013). https://doi.org/10.1038/modpathol.2012.136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2012.136
Keywords
This article is cited by
-
TSC2-mutant uterine sarcomas with JAZF1-SUZ12 fusions demonstrate hybrid features of endometrial stromal sarcoma and PEComa and are responsive to mTOR inhibition
Modern Pathology (2022)
-
JAZF1–SUZ12 endometrial stromal sarcoma forming subserosal masses with extraordinary uptake of fluorodeoxyglucose on positron emission tomography: a case report
Diagnostic Pathology (2019)