Abstract
Epithelial–mesenchymal transition is a physiopathological process by which epithelial cells acquire mesenchymal shape and properties. Malignant mesothelioma is histologically characterized by the concomitant presence of epithelioid and sarcomatoid features, the latter being associated to worse prognosis, thus suggesting a role of epithelial–mesenchymal transition in this dual phenotype. We studied 109 malignant mesotheliomas (58 epithelioid, 26 sarcomatoid, and 25 biphasic) by immunohistochemistry and qRT–PCR analysis, and demonstrated a substantial switch from epithelial markers (E-cadherin, β-catenin, and cytokeratins 5/6) to mesenchymal markers (N-cadherin, vimentin, α-smooth muscle actin, Snail, Slug, Twist, ZEB1, ZEB2, S100A4, MMP2, and MMP9) through epithelioid to biphasic and sarcomatoid histotypes. In agreement with these findings, the ectopic expression of miR-205 (a repressor of ZEB1 and ZEB2 expression) in MeT-5A (mesothelial cell line), H2452 (an epithelioid malignant mesothelioma cell line) and MSTO-211H (a biphasic malignant mesothelioma cell line) not only induced a significant reduction of ZEB1 and ZEB2 and a consequent up-regulation of E-cadherin gene expression, but also inhibited migration and invasion. Moreover, miR-205 was significantly down-regulated in biphasic and sarcomatoid histotypes (qRT–PCR and in situ hybridization analyses). Collectively, our findings indicate that epithelial–mesenchymal transition has a significant part in the morphological features of malignant mesothelioma. In particular, miR-205 down-regulation correlated significantly with both a mesenchymal phenotype and a more aggressive behavior.
Similar content being viewed by others
Main
Epithelial–mesenchymal transition is a cellular process by which epithelial cells lose some of their typical characteristics, such as polarization and immotility, and acquire mesenchymal shape and properties.1, 2 Epithelial–mesenchymal transition has a central role in both physiological (ie growth and wound healing) and pathological processes (ie fibrosis and cancer).3, 4
In cancer, epithelial–mesenchymal transition is involved in many mechanisms, especially invasion and motility, but also in resistance to apoptosis, senescence, immunotolerance, immunosuppression, drug resistance, and the acquisition of stem-cell-like properties.5, 6
Epithelial–mesenchymal transition involves a loss of intercellular adhesion and polarity markers, cytoskeletal reorganization, and degradation of the basement membrane.2 Commonly used molecular markers of epithelial–mesenchymal transition include a reduced expression of E-cadherin, cytokeratins (CK), and β-catenin (in the membrane), and an increased expression of Snail, Slug, Twist, ZEB1, ZEB2, N-cadherin, vimentin, α-smooth muscle actin (αSMA), S100A4, and matrix metalloproteinases (MMP).5, 7
In particular, activation of the transcriptional regulators Snail, Slug, Twist, ZEB1, and ZEB2 enables the changes in gene expression patterns underlying epithelial–mesenchymal transition to be regulated.8, 9, 10, 11, 12 These molecular markers repress the gene expression of E-cadherin, a marker of the epithelial phenotype.7, 8, 9, 13, 14, 15, 16
MicroRNAs (or miRNAs) are a class of short non-coding RNAs that regulate gene expression in several physiological and pathological cellular processes.17, 18 Recently, miR-205 has been identified as a key regulator of epithelial–mesenchymal transition enforcing the epithelial phenotype.19, 20, 21, 22 This miRNA participates directly with ZEB1 and ZEB2 in a signaling network that is postulated to facilitate the maintenance of stable epithelial or mesenchymal states, but that also allows for reversible switching between these states in response to epithelial–mesenchymal transition effectors.19, 20, 21, 22, 23
Malignant mesothelioma is the tumor arising from the cells lining serosal cavities.24 According to the WHO classification, malignant mesothelioma is subclassified as epithelioid (mostly composed of epithelial-shaped cells), sarcomatoid (mostly composed of spindle-shaped cells), or biphasic (composed of both types of cell).24 The morphological patterns of malignant mesothelioma are therefore likely to be the outcome of different steps in an epithelial–mesenchymal transition process.25 Several authors reported the association between malignant mesothelioma subtype and patient prognosis.25, 26, 27, 28, 29 Indeed, a purely epithelioid histology involved the longest survival, a purely sarcomatoid histology the worst, and a biphasic pattern an intermediate survival.26, 27, 28
This study was conducted to investigate the role of epithelial–mesenchymal transition in malignant mesothelioma histological subtyping. Judging from the results obtained, malignant mesothelioma could be considered an original in vivo model for studying the epithelial–mesenchymal transition process.
Materials and methods
Patients and Tissue Specimens
One hundred and nine malignant mesotheliomas (58 epithelioid, 26 biphasic, and 25 sarcomatoid) reported between 2002 and 2009 were retrieved from the archives of the Surgical Pathology and Cytopathology Unit at Padova University. Overall, the male/female ratio was 76/33 and the patients mean age was 68.9±9.9 years (median=69.0; range=47–83). Of these 109 cases, 74 malignant mesotheliomas were of pleural origin and 35 were peritoneal (Table 1). All diagnoses were based on the WHO criteria and confirmed in all instances by two pathologists (AF and MF) on clinical, histological, and immunohistochemical data.24 Written informed consent to the study from the patients was obtained.
Immunohistochemistry and Immunocytochemistry
Immunohistochemical reactions were obtained on 4–5 μm-thick formalin-fixed and paraffin-embedded sections from each tumor sample. All immunohistochemical stains were performed automatically (Bond™—maX, Menarini, Florence, Italy) with the panel of primary antibodies listed in Table 2, according to the manufacturer's instructions. Sections were then lightly counterstained with hematoxylin. For immunocytochemistry, cells were seeded (104 cells/well) on fibronectin-coated cover slides on a 24-well plate, and stained as described elsewhere.30 Appropriate positive and negative controls were run concurrently. E-cadherin and N-cadherin immunoreactions were defined as immunoreactions detectable in the membrane, while for β-catenin, MMP2, and MMP9, the membrane and the cytoplasm were considered, for CK 5/6, vimentin, αSMA, and S100A4 only the cytoplasm, and for ZEB1 and ZEB2 the nucleus. Immunohistochemical reactions were semiquantitatively scored in a four-tier scale: negative=0–5% positive cancer cells, score 1=6–33%, score 2=34–66%, score 3=67–100%. Slides were scored independently by three pathologists (AF, RC, MF) and a consensus score was reached.
In Situ Hybridization
Reactions were obtained on formalin-fixed and paraffin-embedded sections 4–5 μm thick using the GenPointTM Catalyzed Signal Amplification System (DakoCytomation, Glostrup, Denmark) and applying the biotin-labeled miRCURY LNA detection probe (Exiqon, Vedbæk, Denmark) for miR-205 (probe sequence: 5′-cagactccggtggaatgaagga-3′) or the scrambled negative control probe (U6, Exiqon) at 200 nM final concentration, as explained elsewhere.31
Reverse Transcription and Quantitative Real-Time PCR
In all, 2 mm cores of tissue were obtained from the microdissected paraffin block of each tumor sample and deparaffinized with xylene at 50 °C for 3 min. Total RNA was extracted using the RecoverAll kit (Ambion, Austin, TX, USA), as reported elsewhere.32, 33 For cell lines, total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Reverse transcription was done using 100 ng of total RNA, M-MLV Reverse Transcriptase (Invitrogen) and 250 μM random primers (Invitrogen). Quantitative real-time PCR analyses for CDH1 (E-cadherin), CDH2 (N-cadherin), SNAI1 (Snail), SNAI2 (Slug), TWIST1 (Twist), ZEB1, ZEB2, and B2M (β-2-microglobulin) were performed with the LightCycler 480 Real-Time PCR System (Roche Diagnostics, Mannheim, Germany). The primer sequences (shown in Table 3) and the respective probes were designed using the Universal ProbeLibrary (Roche) and the ProbeFinder software (http://www.roche-applied-science.com/). Experiments were performed according to a standard protocol using the LightCycler 480 Probes Master (Roche). B2M was included as housekeeping gene control to adjust for unequal RNA amounts. To detect and quantify mature miR-205 (primer sequence in Table 3), the NCodeTM miRNA qRT–PCR method (Invitrogen) was used on the LightCycler 480 Real-Time PCR System (Roche), according to the manufacturer's instructions, normalizing the results with the small nuclear RNA U6B (RNU6B; Invitrogen). All the reactions were run in triplicate, including no-template controls.
Cell Cultures
The immortalized by SV-40 transfection mesothelial cell line MeT-5A and the H2452 (epithelioid) and MSTO-211H (biphasic) malignant mesothelioma-derived cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained as recommended.
Cell Proliferation Assay
Cells were seeded in triplicate (104 cells/well) into a 24-well plate and cultured in their specific medium, replacing the culture medium every 24 h. Cell proliferation was assessed after 24, 48, 72, and 96 h, by manually counting the cells in the Burker chamber under the microscope using the Trypan Blue exclusion method to count only viable cells.
Migration Assay
Cells were starved of serum for 24 h, then seeded in triplicate (103 in 200 μl) in Boyden chambers (upper chamber) (BD Biosciences, Bedford, MA, USA). Lower chambers contained 500 μl of serum-free medium (SFM) or 500 μl of medium supplemented with 10% fetal bovine serum. After 24 h, the cells in the upper chamber were removed, whereas the cells that migrated to the lower chamber were counted under the microscope after fixing and staining in Coomassie blue solution, as described.34, 35
Invasion Assay
Cell invasion through a three-dimensional extracellular matrix was assessed using a Matrigel invasion assay with BD Matrigel Invasion Chambers (BD Biosciences), as described.34, 35 Briefly, cells were starved of serum for 24 h, then seeded in triplicate (103 in 200 μl) in Boyden chambers (upper chamber) (BD Biosciences). Lower chambers contained 500 μl of SFM or 500 μl of medium supplemented with 10% fetal bovine serum. After 24 h, the cells on the upper surface of the filters were removed with cotton swabs, whereas cells that had invaded to the lower surface of the filter were counted under the microscope after fixing and staining in Coomassie blue solution for 5 min.
Cell Transfection
Cells were seeded (104 cells/well) into a 24-well plate and cultured in their specific medium. After 24 h of incubation, the medium was removed and replaced with 500 μl of transfection medium composed of 2.5 μl DharmaFECT 1 reagent (Dharmacon, Thermo Fisher Scientific, Waltham, MA, USA), 2.5 μl of 5 μM miR-205 miRIDIAN miRNA mimic (double-stranded, chemically modified RNA oligonucleotides; Dharmacon), and 95 μl antibiotic and SFM, with a final concentration of 25 nM miRNA mimic. Transfection of miRNA mimic was confirmed by qRT–PCR for miR-205. Negative miRIDIAN mimics were transfected as matched controls. After 24 h, cells were processed and analyzed for migration and invasion as described above.
Statistical Analysis
All statistical analyses were performed using the R software (R Development Core Team, version 2.9; R Foundation for Statistical Computing, Vienna, Austria). Survival was calculated as the time of diagnosis to the date of death or last contact (after a follow-up of at least 18 months). Survivor curves with censored data were estimated from the univariate Cox model; comparisons between groups were performed using log-rank test. Statistical significance was determined for immunohistochemical and qRT–PCR results using Student's t-test and the Kruskal–Wallis test. A P-value <0.05 was considered statistically significant.
Results
Malignant Mesothelioma Histotypes Are Associated with Different Survival Time
As expected, the median survival time for epithelioid malignant mesothelioma was 13 months (95% CI=13–21 months), for biphasic malignant mesothelioma was 14 months (95% CI=11–27 months), and for sarcomatoid malignant mesothelioma was 6 months (95% CI=6–13 months). Survivor curves for the epithelioid, biphasic, and sarcomatoid histotypes with censored times are showed in Figure 1. The log-rank test revealed differences in survival time for the three histotypes (LR=17.3, P<0.001). The Cox model showed that patients with sarcomatoid malignant mesothelioma had a significantly worse prognosis than those with epithelioid and biphasic malignant mesothelioma (P<0.001), while there was no difference for those with these last subtypes (P=0.146).
Kaplan–Meier survival curves with censored times (crosses) in malignant mesothelioma patients according to the histological subtype. Curves were compared by univariate (log-rank) test, which revealed differences in survival time for the three histotypes (LR=17.3, P<0.001) and Cox model displayed that the survival time of patients with sarcomatoid malignant mesothelioma was significantly shorter than epithelioid and biphasic malignant mesotheliomas (P<0.001), while there was no difference between these last subtypes (P=0.146).
Malignant Mesothelioma Phenotypic Subclassification Corresponds to Specific Expression Profiles of Epithelial–Mesenchymal Transition Markers
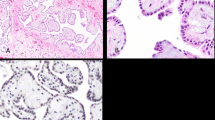
Immunohistochemical analyses were performed on our series of 109 formalin-fixed paraffin-embedded malignant mesothelioma samples. Immunostaining showed that epithelial hallmarks such as E-cadherin and CK 5/6 were positive and reliable markers of epithelioid commitment, while their expression was weak in biphasic and absent in sarcomatoid malignant mesothelioma samples (Figures 2 and 3; P<0.001 for all markers). The β-catenin levels were likewise higher in epithelioid and biphasic histotypes than in sarcomatoid malignant mesotheliomas (Figures 2 and 3). On the other hand, the typical mesenchymal markers vimentin, S100A4, and ZEB1 displayed a strong reaction in all sarcomatoid samples, a moderate reaction in biphasic malignant mesotheliomas, and a weak and focal reaction in epithelioid malignant mesotheliomas (Figures 2, 4, and 5). MMP9, N-cadherin, αSMA, ZEB2, and MMP2 did not differ significantly between biphasic and sarcomatoid malignant mesotheliomas, while they were lower in the epithelioid histotype (Figures 2, 4, and 5). The log-rank test showed that the survival time for the epithelioid malignant mesothelioma was associated with the immunohistochemical level of the epithelial marker E-cadherin (P=0.042). No differences were observed relating to the anatomical site of involvement (pleura or peritoneum). These findings were further supported by qRT–PCR analysis, which partially confirmed the shift from the epithelial to the mixed and mesenchymal phenotypes. Indeed, qRT–PCR revealed the cadherin switch, showing that CDH1 mRNA levels were much higher in epithelioid malignant mesotheliomas than in biphasic and sarcomatoid malignant mesotheliomas, and that CDH2 mRNA levels were markedly increased in the sarcomatoid histotype by comparison with the biphasic or epithelioid histotypes (Figure 6). Analysis of the transcriptional repressors regulating epithelial–mesenchymal transition showed that SNAI2, TWIST1, and ZEB2 mRNA levels were higher in sarcomatoid than in epithelioid samples, and TWIST1 and ZEB2 mRNA levels were higher in sarcomatoid than in biphasic malignant mesotheliomas (Figure 6). Notwithstanding SNAI1 and ZEB1 amounts were not statistically different among the three histotypes, ZEB1 mRNA levels showed an upward trend from epithelioid to biphasic and sarcomatoid malignant mesotheliomas (Figure 6).
Epithelial–mesenchymal transition marker expression in malignant mesotheliomas. Radar charts show the mean immunohistochemical scores observed in epithelioid, biphasic, and sarcomatoid malignant mesotheliomas; center of the chart correspond to negative immunohistochemical reaction while the edges to a score of 3. Note that the epithelial markers CK 5/6, E-cadherin, and β-catenin levels are consistently higher in epithelioid than in sarcomatoid malignant mesotheliomas (P<0.001 for all markers), and in biphasic than in sarcomatoid malignant mesotheliomas (P<0.001 for all markers); and CK 5/6 and E-cadherin are significantly more over-expressed in epithelioid than in biphasic samples (P=0.005 and P=0.028, respectively). Typical mesenchymal markers, such as αSMA, S100A4, vimentin, N-cadherin, ZEB1, ZEB2, MMP2 and MMP9, are significantly more over-expressed in sarcomatoid and biphasic than in epithelioid malignant mesotheliomas (P<0.01 for all markers).
Relative fold of CDH1, CDH2, SNAI1, SNAI2, TWIST1, ZEB1, and ZEB2 mRNA levels in 58 epithelioid (black), 26 biphasic (dark gray), and 25 sarcomatoid (bright gray) malignant mesotheliomas. qRT–PCR highlighted the differences in cadherin levels: CDH1 was significantly higher in epithelioid than in biphasic or sarcomatoid samples (P=0.049 and P=0.039, respectively), while CDH2 was more over-expressed in sarcomatoid than in biphasic or epithelioid malignant mesotheliomas (P=0.009 and P=0.028, respectively). Mesenchymal transcriptional regulators SNAI2, TWIST1, and ZEB2 were consistently more over-expressed in the sarcomatoid than in the epithelioid histotype (P=0.029, P=0.009, and P=0.006, respectively); TWIST1 and ZEB2 mRNA levels were also higher in biphasic than in epithelioid malignant mesotheliomas (P=0.016 and P=0.049, respectively). SNAI1 and ZEB1 amounts were not statistically different among the three histotypes; however, ZEB1 showed an upward trend from epithelioid to biphasic and sarcomatoid malignant mesotheliomas. Columns, representative images of reactions run in triplicate; bars, s.d. *P<0.05; **P<0.01.
Epithelial–Mesenchymal Transition Dysregulation Is a Key Determinant of the Phenotypic and Aggressive Features of Mesothelial/Mesothelioma-Derived Cell Lines
To establish the effect of epithelial–mesenchymal transition on malignant mesothelioma phenotype and behavior, we performed immunocytochemistry, proliferation, migration, and invasion assays in the mesothelial cell line MeT-5A and in the established H2452 (epithelioid) and MSTO-211H (biphasic) cell lines. The immunocytochemical results indicated that the epithelial markers E-cadherin and CK 5/6 were weakly expressed only in the mesothelial cell line MeT-5A, while β-catenin expression was strong in MeT-5A, weak in H2452, and absent in MSTO-211H (Figure 7). Conversely, all mesenchymal markers, αSMA, S100A4, vimentin, N-cadherin, ZEB1, ZEB2, MMP2, and MMP9 occurred in higher levels in the biphasic than in the epithelioid or non-tumorigenic cell lines (Figures 7 and 8). Immunostaining also showed that αSMA, S100A4, vimentin, ZEB2, and MMP2 were expressed more in H2452 than in MeT-5A (Figure 7). Proliferation assays showed that both the malignant mesothelioma cell lines had a considerably greater increase in cell number than MeT-5A, and that the biphasic cells grew faster than all the others (Figure 9). The more aggressive behavior of H2452 and MSTO-211H, compared with MeT-5A, was also confirmed by migration and invasion assays, as shown in Figure 10.
Mesothelial-derived cellular commitment corresponds to specific epithelial–mesenchymal transition marker profiles. Radar charts show the mean immunocytochemical scores in the mesothelial cell line MeT-5A and the established H2452 (epithelioid) and MSTO-211H (biphasic) malignant mesothelioma cell lines. The figures are the mean of three different immunoreactions obtained from three different cell passages; center of the chart correspond to negative immunohistochemical reaction while the edges to a score of 3. Overall, marker expression increased from MeT-5A to H2452 and MSTO-211H.
Mesothelial-derived cellular commitment corresponds to specific epithelial–mesenchymal transition marker profiles. Representative images of immunostaining for the mesenchymal markers N-cadherin, S100A4, ZEB1, and ZEB2 in the three cell lines studied (original magnifications × 40). Overall, marker expression increased from MeT-5A to H2452 and MSTO-211H.
MeT-5A (normal mesothelial cell line) showed a less aggressive behavior than H2452 of MSTO-211H (epithelioid and biphasic malignant mesothelioma cell lines), in both the migration and the invasion assays. Migration and invasion experiments on the three cell lines were done using SFM or medium supplemented with 10% fetal bovine serum. Percent increase over control (SFM). Columns, representative images of triplicates from three independent experiments; bars, s.e.m. *P<0.05; **P<0.01.
MiR-205 Expression Is Dysregulated in the Different Mesothelioma Subtypes and Inhibits Migratory and Invasive Phenotypes
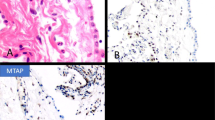
MiR-205 is known to be a negative regulator of ZEB1 and ZEB2 mRNA thus maintaining the epithelial phenotype.19, 20, 21, 22 A qRT–PCR analysis, performed in solid malignant mesotheliomas and in cell lines, showed that miR-205 levels were higher in epithelioid than in biphasic or sarcomatoid samples, and in MeT-5A than in malignant mesothelioma cell lines (Figure 11; P<0.01 for both). To support this observation, a miR-205-specific in situ hybridization was performed in 15 malignant mesotheliomas corresponding to the three different phenotypic forms (five cases of each subtype), randomly selected from the immunohistochemical series. In situ hybridization displayed a strong and diffuse reaction in the epithelioid samples, a moderate reaction in the biphasic samples, and a weak and focal reaction in the sarcomatoid samples (Figure 12). To assess the effect of miR-205 dysregulation on cellular behavior, mesothelial/malignant mesothelioma-derived cell lines were transfected with a miR-205 mimic. In treated and untreated cells, in all the cell lines, qRT–PCR analysis revealed a consistent down-regulation of ZEB1 and ZEB2 and a slight up-regulation of CDH1 coinciding with the increase of miR-205 levels (Figure 13). To investigate the role of miR-205 in cell migratory and invasive phenotypes, we conducted in vitro migration and invasion assays using the biphasic MSTO-211H cell line: miR-205 over-expression inhibited the cells’ capacity of migration and invasion, indicating that miR-205 negatively regulates the metastatic phenotype of mesothelioma cancer cells (Figure 14). We repeated these assays in the other two cell lines and obtained similar results, albeit without reaching statistical significance (data not shown).
MiR-205 is key factor of epithelial–mesenchymal transition in malignant mesothelioma. The relative expression of miR-205 was analyzed in both solid malignant mesotheliomas and mesothelial cell line MeT-5A, established H2452 (epithelioid) and MSTO-211H (biphasic) malignant mesothelioma cell lines. qRT–PCR analysis showed that miR-205 levels were significantly higher in epithelioid than in biphasic or sarcomatoid samples (P<0.01 for both), and in non-tumorigenic and epithelioid than in biphasic cell lines (P<0.01 for both). Columns, representative images of reactions run in triplicate; bars, s.d. **P<0.01.
MiR-205 regulated ZEB1 and ZEB2 and consequently CDH1 expression in MeT-5A, H2452, and MSTO-211H cell lines. Cells were transfected with miR-205 mimic or a negative control (scramble) and analyzed by qRT–PCR. Columns, representative images of reactions run in triplicate; bars, s.d. *P<0.05; **P<0.01.
Transfection with miR-205 mimic inhibited the migratory and invasive properties of MSTO-211H cancer cells. Migration and invasion experiments on MSTO-211H cancer cells were done using SFM or medium supplemented with 10% fetal bovine serum. Percent increase over SFM. Columns, representative images of triplicates from three independent experiments; bars, s.e.m. **P<0.01.
Discussion
The current histological classification recognizes three major subtypes of malignant mesothelioma: epithelioid malignant mesothelioma is composed of epithelial-shaped cells, sarcomatoid malignant mesothelioma consists of cells with a mesenchymal appearance, and biphasic malignant mesothelioma is a combination of the two.24 This phenotypic triad reflects the whole spectrum of cell commitments occurring during the epithelial–mesenchymal transition process, and this was the working hypothesis behind the present study, in which we investigated the role of epithelial–mesenchymal transition in malignant mesothelioma histological subtyping.
In a large series of malignant mesotheliomas, we observed a consistent epithelial-to-mesenchymal trend in marker representation coinciding with the epithelioid to biphasic, to sarcomatoid histotypes by immunohistochemistry and qRT–PCR analysis. In the sarcomatoid samples, E-cadherin virtually disappeared, as reported in other solid tumors undergoing epithelial–mesenchymal transition.36, 37, 38 This crucial passage coincided with an increased expression of Slug, Twist, ZEB1, and ZEB2, a set of well-known nuclear factors that inhibit E-cadherin gene transcription by binding to its promoter.5, 7, 19, 21, 22
E-cadherin has recently been found to be regulated at protein level by mechanisms implicating miRNAs, a class of small non-coding RNAs that modulate gene expression post-transcriptionally.19, 20, 21, 22 Indeed, it has been demonstrated in vitro that miR-205 controls ZEB1 and ZEB2 levels by targeting their messenger RNA, thus enhancing E-cadherin expression and maintaining the epithelial phenotype.19
Based on these data, we found that changes in E-cadherin expression varied according to the miR-205 levels in the different histotypes. Moreover, when miR-205 was over-expressed, a significant ZEB1/ZEB2 down-regulation and a minor CDH1 up-regulation were consistently seen in the mesothelial/mesothelioma-derived cell lines.
The other proteins involved in epithelial–mesenchymal transition mechanisms (implicated in cell–cell and cell–matrix interactions, cell structure, and motility) also showed a different expression profile for the three different malignant mesothelioma subtypes. In addition to E-cadherin, epithelioid malignant mesotheliomas expressed β-catenin and CK 5/6, which are needed to maintain cell contact and epithelial shape.39, 40 On the other hand, biphasic and sarcomatoid malignant mesothelioma variants lost these proteins and gained a mesenchymal phenotype by (i) increasing the amount of N-cadherin on the cell surface, which results in weaker cell adhesion; (ii) expressing vimentin, αSMA, and S100A4 in the cytoskeleton, achieving a resistance to deformation and motility; and (iii) producing MMP2 and MMP9 to degrade the basement membrane.1, 2, 3, 5, 7 In this study, however, we evaluated only the loss or acquisition of marker physiological expression and localization, while other mechanisms involved in epithelial–mesenchymal transition, such as the nuclear localization of β-catenin and S100A4, were not considered.
Epithelial–mesenchymal transition is reported to be implicated in many tumoral mechanisms, mainly invasion and motility, which lead to a more aggressive behavior of tumors and, consequently, to a worse prognosis for patients.5, 6 As reported by several authors, our data confirmed the association between sarcomatoid malignant mesothelioma and worst outcome, once again supporting the hypothesis of an epithelial–mesenchymal transition involvement.25, 26, 27, 28 Moreover, our findings highlighted the link between the epithelial marker E-cadherin and the longer survival time of epithelioid malignant mesothelioma than the other subtypes. Further larger studies should investigate the possible correlations of the epithelial–mesenchymal transition markers with survival among the histological variants of epithelioid malignant mesothelioma (and the pleomorphic subtype, in particular) and also with the presence/absence of lymph node metastasis.29
Overall, the demonstration of epithelial–mesenchymal transition in malignant mesothelioma is a suitable model for studying this process in vivo. One of the most important arguments against the existence of epithelial–mesenchymal transition in human solid tumors is that it has not been identified in most cases.40, 41 This could be due to the clonal heterogeneity of epithelial–mesenchymal transition in primary tumors, that is, cells that have undergone epithelial–mesenchymal transition are just a subpopulation located in certain neoplastic areas, and the process could be difficult to recognize using routine histopathological investigations.42 Another reported reason for the difficulty encountered in identifying this process in vivo is the reverse mesenchymal–epithelial transition process occurring in metastatic tumors.43 In contrast, malignant mesothelioma histotype subtyping is relatively straightforward using a suitable immunohistochemical panel, and malignant mesothelioma is therefore a reasonably easy model to study.
Our findings on the migratory and invasive capacity of malignant mesothelioma cell lines confirm the link between cellular phenotype and behavior, as reported by many other authors in other neoplastic diseases.1, 2, 3, 5, 7 As expected, the biphasic malignant mesothelioma cell line showed a more aggressive behavior, in terms of both cell growth and migration/invasion, than MeT-5A and epithelioid malignant mesothelioma cell lines. The ectopic expression of miR-205 also contributed to the mesenchymal–epithelial transition by (i) reducing ZEB1 and ZEB2 expression and consequently increasing CDH1 expression and (ii) interfering with cell motility and invasiveness.19
Based on the present study, we conclude that the different morphological patterns of malignant mesothelioma are part of a continuous spectrum determined by epithelial–mesenchymal transition, initiated by ZEB1 and ZEB2 activation and correlated with a worse outcome. In particular, we found that loss of miR-205 expression correlated significantly with both a mesenchymal phenotype and a more aggressive behavior. Malignant mesothelioma may be a powerful model for studying epithelial–mesenchymal transition in vivo. Regulating epithelial–mesenchymal transition marker expression might be a novel strategy for the chemoprevention of metastatic/aggressive human cancers.
References
Thiery JP, Acloque H, Huang RY, et al. Epithelialmesenchymal transitions in development and disease. Cell 2009;139:871–890.
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420–1428.
Acloque H, Adams MS, Fishwick K, et al. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 2009;119:1438–1449.
Yang J, Weinberg RA . Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 2008;14:818–829.
Voulgari A, Pintzas A . Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 2009;1796:75–90.
Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133:704–715.
Zeisberg M, Neilson EG . Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009;119:1429–1437.
Nieto MA . The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002;3:155–166.
Hajra KM, Chen DY, Fearon ER . The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res 2002;62:1613–1618.
Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004;117:927–939.
Vandewalle C, Van Roy F, Berx G . The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci 2009;66:773–787.
Brabletz S, Brabletz T . The ZEB/miR-200 feedback loop—a motor of cellular plasticity in development and cancer? EMBO Rep 2010;11:670–677.
Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000;2:76–83.
Eger A, Aigner K, Sonderegger S, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 2005;24:2375–2385.
Sanchez-Tillo E, Lazaro A, Torrent R, et al. ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene 2010;29:3490–3500.
Casas E, Kim J, Bendesky A, et al. Snail2 is an essential mediator of twist1-induced epithelial mesenchymal transition and metastasis. Cancer Res 2011;71:245–254.
Bartel DP . MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–297.
Fassina A, Cappellesso R, Fassan M . Classification of non-small cell lung carcinoma in trans-thoracic needle specimens using microRNA expression profiling. Chest 2011; doi:10.1378/CHEST.11-0708 (in press).
Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593–601.
Gregory PA, Bracken CP, Bert AG, et al. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle 2008;7:3112–3118.
Korpal M, Lee ES, Hu G, et al. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem 2008;283:14910–14914.
Bracken CP, Gregory PA, Kolesnikoff N, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 2008;68:7846–7854.
Inui M, Martello G, Piccolo S . MicroRNA control of signal transduction. Nat Rev Mol Cell Biol 2010;11:252–263.
Travis WD, Brambilla E, Muller-Hermelink H, et al. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. IARC Press: Lyon, 2004, pp 125–144.
Schramm A, Opitz I, Thies S, et al. Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma. Eur J Cardiothorac Surg 2010;37:566–572.
Rusch VW, Venkatraman ES . Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg 1999;68:1799–1804.
Zellos LS, Sugarbaker DJ . Multimodality treatment of diffuse malignant pleural mesothelioma. Semin Oncol 2002;29:41–50.
Milano MT, Zhang H . Malignant pleural mesothelioma: a population-based study of survival. J Thorac Oncol 2010;5:1841–1848.
Kadota K, Suzuki K, Sima CS, et al. Pleomorphic epithelioid diffuse malignant pleural mesothelioma: a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol 2011;6:896–904.
Albertin G, Guidolin D, Sorato E, et al. Pro-angiogenic activity of Urotensin-II on different human vascular endothelial cell populations. Regul Pept 2009;157:64–71.
Fassan M, Volinia S, Palatini J, et al. MicroRNA expression profiling in human Barrett's carcinogenesis. Int J Cancer 2011;129:1661–1670.
Fassan M, Baffa R, Palazzo JP, et al. MicroRNA expression profiling of male breast cancer. Breast Cancer Res 2009;11:R58.
Baffa R, Fassan M, Volinia S, et al. MicroRNA expression profiling of human metastatic cancers identifies cancer gene targets. J Pathol 2009;219:214–221.
Monami G, Gonzalez EM, Hellman M, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res 22006;66:7103–7110.
Lovat F, Bitto A, Xu SQ, et al. Proepithelin is an autocrine growth factor for bladder cancer. Carcinogenesis 2009;30:861–868.
Mandal M, Myers JN, Lippman SM, et al. Epithelial to mesenchymal transition in head and neck squamous carcinoma: association of Src activation with E-cadherin down-regulation, vimentin expression, and aggressive tumor features. Cancer 2008;112:2088–2100.
Nguyen PT, Kudo Y, Yoshida M, et al. N-cadherin expression is correlated with metastasis of spindle cell carcinoma of head and neck region. J Oral Pathol Med 2011;40:77–82.
Schwock J, Bradley G, Ho JC, et al. SNAI1 expression and the mesenchymal phenotype: an immunohistochemical study performed on 46 cases of oral squamous cell carcinoma. BMC Clin Pathol 2010;10:1.
Cates JM, Dupont WD, Barnes JW, et al. Markers of epithelial-mesenchymal transition and epithelial differentiation in sarcomatoid carcinoma: utility in the differential diagnosis with sarcoma. Appl Immunohistochem Mol Morphol 2008;16:251–262.
Tarin D, Thompson EW, Newgreen DF . The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res 2005;65:5996–6000.
Christiansen JJ, Rajasekaran AK . Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 2006;66:8319–8326.
Scheel C, Onder T, Karnoub A, et al. Adaptation versus selection: the origins of metastatic behavior. Cancer Res 2007;67:11476–11479; discussion 11479–11480.
Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003;4:915–925.
Acknowledgements
We are grateful to Dr Marta Meneghetti for her help and to the members of Dalla Via laboratory, especially Dr Alessia Oppio and Dr Valentina Graffiti, for their technical assistance with the cell cultures. This work was supported by a grant from the Veneto Region (Ricerca Finalizzata 2006) and by the Cariparo Foundation Excellence grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Fassina, A., Cappellesso, R., Guzzardo, V. et al. Epithelial–mesenchymal transition in malignant mesothelioma. Mod Pathol 25, 86–99 (2012). https://doi.org/10.1038/modpathol.2011.144
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2011.144
Keywords
This article is cited by
-
Micro-RNA-215 and -375 regulate thymidylate synthase protein expression in pleural mesothelioma and mediate epithelial to mesenchymal transition
Virchows Archiv (2022)
-
Metabolic rewiring and redox alterations in malignant pleural mesothelioma
British Journal of Cancer (2020)
-
Hypoxia promotes acquisition of aggressive phenotypes in human malignant mesothelioma
BMC Cancer (2018)
-
DNA methylation profiling of asbestos-treated MeT5A cell line reveals novel pathways implicated in asbestos response
Archives of Toxicology (2018)
-
Cathepsin L upregulation-induced EMT phenotype is associated with the acquisition of cisplatin or paclitaxel resistance in A549 cells
Acta Pharmacologica Sinica (2016)