Abstract
Synovial sarcoma is classified as a tumor of uncertain differentiation, and some synovial sarcomas have rhabdoid cells. In previous studies, all malignant rhabdoid tumors and renal medullary carcinomas, some extraskeletal myxoid chondrosarcomas, almost all epithelioid sarcomas and half of epithelioid malignant peripheral nerve sheath tumors showed a loss of SMARCB1/INI1 protein expression in tumor cells and all of these tumors are also known to have rhabdoid cells. We analyzed the immunohistochemical and mRNA expression of SMARCB1/INI1 in 95 synovial sarcomas (73 monophasic fibrous type, 18 biphasic type and 4 poorly differentiated type) and 30 spindle cell sarcomas (3 adult fibrosarcomas, 7 fibrosarcomas arising in dermatofibrosarcoma protuberans, 10 leiomyosarcomas and 10 malignant peripheral nerve sheath tumors) resembling monophasic fibrous synovial sarcoma. The results have shown that 66 of the 95 synovial sarcoma cases (69%) had reduced SMARCB1/INI1 protein expression, whereas the remaining 29 cases (31%) and all 30 spindle cell sarcomas showed preserved this protein expression. No case with a complete loss of SMARCB1/INI1 protein expression was recognized. The median values of SMARCB1/INI1 mRNA expression in non-tumor skeletal muscle and synovial sarcoma with reduced protein expression were 12.86 and 134.01, respectively, and a statistically significant difference was detected between these two groups (P=0.0000004). However, there was no statistically significant difference of prognosis between the synovial sarcoma group with reduced and that with preserved SMARCB1/INI1 protein expression (P=0.46). Therefore, it was suggested that there is a post-transcriptional SMARCB1/INI1 regulatory mechanism in the tumor cells of synovial sarcoma.
Similar content being viewed by others
Main
Synovial sarcoma, which has been classified as a tumor of uncertain differentiation, accounts for between 5.6 and 10% of adult soft-tissue sarcomas and mainly affects patients between the second and fifth decades of life.1, 2 Histologically, this tumor has three major subtypes, the monophasic type, the biphasic type and the poorly differentiated type.1, 3 Cytogenetic studies frequently show chromosomal translocation t(X;18)(p11.2;q11.2) in this unique tumor. The molecular genetic analysis of the t(X;18) breakpoint has shown that the SS18 gene from chromosome 18 is disrupted and juxtaposed to either SSX1, SSX2 or SSX4 on chromosome X, in a mutually exclusive manner.1, 4, 5, 6, 7
The SMRRCB1/INI1 (INI1) gene is a member of the ATP-dependent SWI/SNF chromatin-remodeling complex, suggesting it is a candidate tumor suppressor gene in malignant rhabdoid tumor.8, 9, 10, 11, 12 Loss of INI1 protein expression has been reported to occur in all malignant rhabdoid tumors and renal medullary carcinomas, almost all epithelioid sarcomas, half of epithelioid malignant peripheral nerve sheath tumors and some extraskeletal myxoid chondrosarcomas.9, 10, 11, 12, 13, 14, 15, 16, 17 These tumors are known to have rhabdoid cells, which are characterized by the existence of a large eosinophilic inclusion within the cytoplasm, eccentric nuclei and prominent nucleoli. Some synovial sarcoma cases also have been reported to possess such rhabdoid features.18 It would therefore be of interest to evaluate INI1 expression in synovial sarcomas, in spite of the fact that there has been only one study on this topic, and that study included only a very small number of synovial sarcomas.16
In this study, we analyzed immunohistochemical INI1 protein expression in a large series of synovial sarcoma cases. In addition, we examined the mRNA expression of INI1 in frozen samples using quantitative reverse transcriptase-polymerase chain reaction (RT-PCR).
Materials and methods
Patients
Ninety-five formalin-fixed, paraffin-embedded specimens of synovial sarcomas, registered at the Department of Anatomic Pathology between 1980 and 2009, were available for this study. The synovial sarcomas consisted of 73 tumors of monophasic fibrous type, 18 of biphasic type and 4 of poorly differentiated type. Frozen materials for quantitative RT-PCR analysis were available in 29 cases (19 monophasic fibrous type, 8 biphasic type and 2 poorly differentiated type). In addition, we also examined 30 spindle cell sarcomas resembling monophasic fibrous synovial sarcomas as controls for the immunohistochemical analysis, and 15 tumors with loss of INI1 protein expression and 20 samples of surrounding non-tumorous skeletal muscle that were collected from patients with various types of sarcoma as controls for the quantitative RT-PCR analysis. The 30 spindle cell sarcomas consisted of 3 adult fibrosarcomas, 7 fibrosarcomas arising in dermatofibrosarcoma protuberans, 10 leiomyosarcomas and 10 malignant peripheral nerve sheath tumors, and the 15 tumors with loss of INI1 protein expression were made up of 9 malignant rhabdoid tumors with INI1 gene alteration at the DNA level causing loss of INI1 protein expression and 6 distal-type epithelioid sarcomas without INI1 gene alteration; all 15 of the tumors with loss of INI1 protein expression were confirmed in our previous study.9, 10 Each sample was prepared from a different patient and all tissues were obtained with the informed consent of the patient. In all cases, the diagnosis was based on light microscopic examination with hematoxylin-eosin staining according to the most recent WHO classification.1, 19, 20, 21, 22, 23, 24 Moreover, immunoperoxidase procedures using the streptavidin-biotin peroxidase method were carried out when necessary.
Immunohistochemistry
Immunohistochemical analyses were performed in all cases, using a streptavidin-biotin-peroxidase method (Histofine; Nichirei, Tokyo, Japan). The primary monoclonal antibody used in this study was BAF47, an antibody to the INI1 gene product (clone 25; 1:250; 20 min microwave; BD Transduction Laboratories, San Diego, CA, USA). Non-tumor tissues, including entrapped normal tissue, inflammatory cell tissue and endothelial cell tissue, were used as a positive control. Immunoreactivity to BAF47 was classified into three categories: −, loss of expression (no staining of tumor nuclei); ±, reduced expression (low-intensity staining of tumor nuclei) compared with the positive control; +, preserved expression (iso-intensity staining of the nuclei) compared with the positive control. Three pathologists (KK, OY and HY) independently evaluated the immunohistochemical staining for each sample.
Western Blot Analysis
Protein was extracted from available 10 frozen samples (nine synovial sarcomas and one leiomyosarcoma) and 2 malignant rhabdoid tumor with loss of INI1 protein expression cell lines (TTC549 and TM87-16) as external negative control, using lysis buffer (PRO-PREP Protein Extraction Solution, iNtRON Biothechnology, Seongnam, Korea) according to the manufacturer's instructions.10 From each sample, 15 μg of protein was run on a 4% to 12% gradient Bis-Tris-HCl buffered (pH 6.4) polyacrylamide gel (NuPAGE Novex 4–12% Bis-Tris Gel, Invitrogen, Life Technologies, Carlsbad, CA, USA). For immunodetection, BAF47, an antibody to the INI1 protein (clone 25; 1:100; BD Transduction Laboratories), and Actin, an antibody to the human actin protein (clone C4; 1:4000 dilution; Millipore, Billerica, MA, USA), were used. Protein expression levels were quantified by image analyzer (LAS-4000 mini, Fujifilm, Tokyo, Japan) and densitometric analysis with Image Gauge software (Fujifilm). The obtained data were standardized by using data of Actin expression level. The final numerical ratio (R) in each sample was calculated as follows: R=(INI1 expression level/Actin expression level) × 100.
RNA Extraction
Total RNA was extracted from frozen and paraffin-embedded samples using Trizol reagent (Invitrogen) according to the manufacturer's instructions. Five micrograms of RNA from each sample was reverse-transcribed using Superscript III reverse transcriptase (Invitrogen) in order to prepare the first-strand cDNA.
Chromosomal Translocation Analysis
Frozen materials were available in 29 patients to detect the SS18-SSX fusion gene transcript. Moreover, formalin-fixed paraffin-embedded materials were available for assay of the SS18-SSX fusion gene transcript in an additional 66 patients. This assay was based on previously reported primers that specifically amplify the fusion gene transcripts of SS18-SSX1 and SS18-SSX2.25 Each PCR product (10 μl) was directly loaded onto 2% agarose gel, stained with ethidium bromide, and directly visualized under UV illumination. The PCR products were evaluated by a direct sequence analysis, and the consistency of those results was confirmed.
TaqMan PCR to Quantify SMARCB1/INI1 mRNA
Quantitative RT-PCR for INI1 was performed using predeveloped TaqMan assay reagents (INI1 Hs00268260_m1; GAPDH Hs99999905_m1; all from Applied Biosystems, Foster City, CA, USA) and an ABI Prism 7700 Sequence Detection system (Applied Biosystems). The PCR reaction was carried out according to the manufacturer's protocol. The standard curve was constructed with serial dilutions of one of the cDNA samples of human normal skeletal muscle. The obtained data were standardized by using data of the internal housekeeping gene, GAPDH. The final numerical value (V) in each sample was calculated as follows: V=(INI1 mRNA value/GAPDH mRNA value) × 10 000.
Results
Fusion Gene Transcript Findings
Among the 29 patients for whom frozen materials were available, 17 and 9 patients showed the SS18-SSX1 and SS18-SSX2 fusion-type genes, respectively. In the remaining three patients these fusion gene transcripts were not detectable. Among the 66 patients for whom only formalin-fixed paraffin-embedded materials were available, large quantities of high-quality total RNA suitable for RT-PCR analysis could be obtained in 18 patients. A fusion-type gene was detected in 14 of these 18 patients. Six patients showed the SS18-SSX1 fusion type, whereas eight patients showed the SS18-SSX2 fusion type.
SMARCB1/INI1 Immunoreactivity
The results of the immunohistochemical analysis are summarized in Table 1. In 66 of the 95 synovial sarcomas (46 cases of monophasic fibrous type, 17 cases of biphasic type and 3 cases of poorly differentiated type), reduced expression of the INI1 gene product was recognized in all tumor cells, vs the level in positive control samples such as infiltrating lymphocytes and entrapped normal tissue (Figure 1). However, in the remaining 29 synovial sarcomas (27 cases of monophasic fibrous type, 1 case of biphasic type and 1 case of poorly differentiated type) and all of the 30 spindle cell sarcomas, the expression of the INI1 gene product in tumor cells was preserved (Figures 1 and 2). None of the cases showed a complete loss of INI1 gene product expression. As for the histological subtype, reduced expression of INI1 was found significantly more frequently in the biphasic type (17/18: 94%) than in the monophasic fibrous type (46/73: 63%) tumors (biphasic vs monophasic, P=0.007; biphasic vs poorly differentiated, P=0.34; monophasic vs poorly differentiated, P=0.54). No statistically significant differences were observed between INI1 immunoreactivity and fusion gene subtype (P=0.44).
Histological and SMARCB1/INI1 immunohistochemical features of synovial sarcoma. (a, b) Monophasic fibrous type (22-year-old female; abdominal wall). (c, d) Biphasic type (20-year-old female; knee). The tumor cells showed reduced expression of SMARCB1/INI1 protein compared with the positive control, which included infiltrating lymphocytes and entrapped normal tissue (b, d). (e, f) Monophasic fibrous type (61-year-old male; thigh). While on the other hand, the tumor cells showed preserved expression of SMARCB1/INI1 protein compared with the positive control (f).
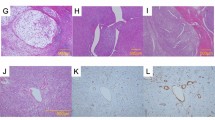
Spindle cell sarcomas resembling monophasic fibrous type of synovial sarcoma. (a, b) Adult fibrosarcoma (37-year-old male; lower thigh). (c, d) Malignant peripheral nerve sheath tumor (25-year-old female; retroperitoneum). (e, f) Leiomyosarcoma (65-year-old female; thigh). All cases show preserved SMARCB1/INI1 expression compared with inflammatory cells and endothelial cells.
Rhabdoid cells were recognized in 9 out of 95 synovial sarcoma cases. These rhabdoid cells possessed cytoplasmic eosinophilic and glassy inclusion bodies, which are essentially identical to the features of malignant rhabdoid tumor. Five out of these nine cases showed reduced expression of the INI1 gene product. However, there was no statistically significant correlation between INI1 immunoreactivity and the presence of rhabdoid features (P=0.91) (Table 2, Figure 3).
Histologic findings and SMARCB1/INI1 immunohistochemical reactivity of monophasic fibrous type with rhabdoid features (17-year-old female; groin; SS18-SSX2 positive). (a) Short spindle-shaped cells were arranged in sheets or fascicles without glandular components. (b) Rhabdoid cells with cytoplasmic inclusion bodies were prominently observed. (c) Immunohistochemically, the tumor cells (rhabdoid and non-rhabdoid cells) showed reduced expression of SMARCB1/INI1 protein compared with entrapped normal cells.
SMARCB1/INI1 Protein Expression Levels
The results of INI1 protein levels are summarized in Figure 4. Expression ratios of leiomyosarcoma case (LS-3) as positive control and malignant rhabdoid tumor cell lines (TTC549 and TM87-16) as negative control are 100, 7.5 and 3.2, respectively. In synovial sarcoma, expression ratios of not only reduced immunohistochemical expression cases (P2-1, M1-2, M2-3, B1-4, B1-5 and M2-6) but also preserved immunohistochemical expression cases (M1-12, M1-13 and M1-14) are lower than that of leiomyosarcoma case.
INI1 protein expression as assessed by western blot. Expression ratios of malignant rhabdoid cell lines (TTC549 and TM87-16) as negative control are 7.5 and 3.2, respectively. Ratios of all synovial sarcoma cases (P2-1; 45.3, M1-2; 56.4, M2-3; 74.4, B1-4; 65.0, B1-5; 40.0, M2-6; 58.7, M1-12; 60.4, M1-13; 58.9 and M1-14; 34.0) were reduced, compared with that of leiomyosarcoma case as positive control (LS-3; 100).
SMARCB1/INI1 mRNA Expression by TaqMan PCR
The analyzed synovial sarcoma cases were divided into two groups according to the results of immunohistochemistry: a group showing reduced expression of the INI1 gene product and a group showing preserved expression of the INI1 gene product. Figure 5 shows the boxplots of INI1 mRNA expression (non-tumor skeletal muscle group, median value=12.86; reduced group, 134.01; preserved group, 91.31; malignant rhabdoid tumor group, 6.19; distal-type epithelioid sarcoma group, 40.52). Paradoxically, INI1 mRNA expression levels of the reduced group and preserved group were significantly higher than that of the non-tumor skeletal muscle group (P=0.0000004, P=0.0003). However, there was no statistically significant difference between the reduced and preserved groups (P=0.24).
Boxplot of SMARCB1/INI1 mRNA expression. The median values of SMARCB1/INI1 mRNA expression in non-tumor skeletal muscle (N muscle), synovial sarcoma with reduced protein expression (INI1±), synovial sarcoma with preserved protein expression (INI1+), malignant rhabdoid tumor (MRT) and distal-type epithelioid sarcoma (ES) were 12.86, 134.01, 91.31, 6.19 and 40.52, respectively (non-tumor skeletal muscle vs synovial sarcoma with reduced protein expression, P=0.0000004; non-tumor skeletal muscle vs synovial sarcoma with preserved protein expression, P=0.0003; synovial sarcoma with reduced protein expression vs preserved protein expression, P=0.24).
Prognosis of Synovial Sarcoma According to SMARCB1/INI1 Protein Expression
Follow-up data were available in 88 of 95 cases (61 reduced INI1 protein expression cases and 27 preserved INI1 protein expression cases). However, there was no statistically significant difference in prognosis between the reduced and preserved cases (P=0.46, Figure 6).
Discussion
Previous immunohistochemical studies have shown that loss of INI1 is a sensitive and specific marker for the diagnosis of malignant rhabdoid tumor, and that complete loss of INI1 protein expression is quite rare in other tumors.10, 16, 17 Nonetheless, loss of INI1 protein expression in other tumors is not unknown.9, 11, 14, 15, 26 In a previous study, for example, a few synovial sarcoma cases showed variable and focal nuclear staining for INI1 protein, and there was no differential staining between the spindle cells and the epithelioid components of these tumors.16 In this study, the vast majority of synovial sarcoma cases (66 of 95 cases; 69%) showed reduced immunohistochemical expression of INI protein, compared with non-tumor cells such as vascular endothelial cells and inflammatory cells. Moreover, in western blot analysis, all of the three preserved immunohistochemical expression cases also showed reduced protein levels, compared with leiomyosarcoma as positive control.
At the present time, the common representative diagnostic factors of synovial sarcoma are morphological arrangements of the tumor cells, cytokeratin and epithelial membrane antigen immunoreactivity, and specific fusion gene transcripts.1 However, when molecular genetic analysis is not available and immunoreactivities for epithelial membrane antigen and cytokeratins are inconspicuous, differential diagnosis from other spindle cell sarcomas might be difficult.27 Poorly differentiated synovial sarcoma cells show even more limited expression of cytokeratin.28, 29 In this study, cases with reduced expression were recognized more frequently among monophasic fibrous-type tumors (63%) than among tumors resembling spindle cell sarcomas (0/30: 0%). Moreover, most poorly differentiated cases (75%) also showed reduced INI1 protein expression. Therefore, evaluation of INI1 protein expression has the potential to become an ancillary parameter in the differential diagnosis of synovial sarcoma. However, to clarify the utility of this diagnostic parameter, further studies using a larger number of the cases will be needed.
In malignant tumors with loss of INI1 protein expression, rhabdoid features are occasionally reported.9, 11, 14, 15, 26 Therefore, the loss of INI1 protein expression may be associated with rhabdoid features. Although synovial sarcomas with rhabdoid features have occasionally been reported, none of the synovial sarcoma cases with rhabdoid features in this study showed a complete loss of INI1 protein expression. However, approximately half of the synovial sarcoma cases (including five of the nine cases with rhabdoid features) showed a reduction of INI1 protein expression. There was no significant correlation between rhabdoid features and INI1 protein expression (P=0.91). Therefore, INI1 protein expression may be associated with a certain histologic tumor type rather than with rhabdoid features.
In the previous studies, in spite of the morphologic differences in brain tumors, tumors with loss of INI1 protein expression shared similar clinical characteristics of an unfavorable outcome with malignant rhabdoid tumor.13 Meanwhile, some investigations have shown that INI1 immunoreactivity may not be related to clinical outcome.9, 11 In this study, the status of INI protein expression did not affect the prognosis of patients with synovial sarcoma (P=0.46), although no cases showed a complete loss of INI1 protein expression. Therefore, INI1 expression may have limited efficacy for the prognosis of synovial sarcoma, but may be more useful for identifying histological tumor categories.
As for INI1 mRNA expression, it was a predictable result that the expression in malignant rhabdoid tumors was lower than that in non-tumor skeletal muscle, because INI1 gene alteration at the DNA level caused a loss of INI1 protein expression. However, in distal-type epithelioid sarcomas, in spite of the loss of INI1 protein expression, the INI1 mRNA expression level was higher than that of non-tumor skeletal muscle. Furthermore, the group of synovial sarcomas with a reduction in INI1 protein expression also showed a higher level of mRNA expression. Therefore, in distal-type epithelioid sarcomas and synovial sarcomas with reduced INI1 protein expression, INI1 protein expression may be regulated by other post-transcriptional regulatory mechanisms such as microRNA. Meanwhile, in synovial sarcoma group with preserved INI1 protein expression, it was suggested that there was another regulatory mechanism or no post-transcriptional regulatory mechanism for INI1, because the INI1 mRNA expression level was not significantly different between the reduced and preserved groups.
In this study, we could not clarify the precise molecular mechanisms of the reduced INI1 protein expression in synovial sarcoma. However, a control through the chromatin-remodeling pathway has been suggested to be one of the mechanisms of synovial sarcoma tumorigenesis in previous studies: the N-terminal amino acids of SS18 bind to BRM, which is one of the components of SWI/SNF complexes, histone acetyltransferase p300, and AF10 (acute lymphoblastic leukemia fused gene from chromosome 10).30, 31, 32, 33, 34 In particular, AF10 through the binding on GAS41 (glioma-amplified sequence 41) indirectly interacts with INI1.31 Therefore, the reduced expression of INI1 proteins, including INI1, may be caused by SS18-SSX post-transcriptional interactions of the chromatin-remodeling pathway.
In summary, we analyzed the INI1 protein expression status in synovial sarcoma and also analyzed the INI1 mRNA expression. Sixty-six of the 95 synovial sarcoma cases showed reduced INI1 protein expression in the immunohistochemical analysis. However, the INI1 mRNA expression level in the group of synovial sarcomas with a reduction in this protein expression was higher than that in non-tumor skeletal muscle group. Therefore, both the previous studies and our present findings suggest that there is a post-transcriptional INI1 regulatory mechanism through SS18-SSX.
References
Fisher C, de Bruijn DRH, van Kessel AG . Synovial sarcoma. In: Fletcher CDM, Unni KK, Mertens F (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, France, 2002, pp 200–204.
Oda Y, Ohishi Y, Saito T, et al. Nuclear expression of Y-box-binding protein-1 correlates with P-glycoprotein and topoisomerase II alpha expression, and with poor prognosis in synovial sarcoma. J Pathol 2003;199:251–258.
Izumi T, Oda Y, Hasegawa T, et al. Dysadherin expression as a significant prognostic factor and as a determinant of histologic features in synovial sarcoma: special reference to its inverse relationship with E-cadherin expression. Am J Surg Pathol 2007;31:85–94.
Clark J, Rocques PJ, Crew AJ, et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet 1994;7:502–508.
Crew AJ, Clark J, Fisher C, et al. Fusion of SYT to two genes, SSX1 and SSX2, encoding proteins with homology to the Kruppel-associated box in human synovial sarcoma. EMBO J 1995;14:2333–2340.
de Leeuw B, Balemans M, Weghuis DO, et al. Molecular cloning of the synovial sarcoma-specific translocation (X;18)(p11.2;q11.2) breakpoint. Hum Mol Genet 1994;3:745–749.
Skytting B, Nilsson G, Brodin B, et al. A novel fusion gene, SYT-SSX4, in synovial sarcoma. J Natl Cancer Inst 1999;91:974–975.
Imbalzano AN, Jones SN . Snf5 tumor suppressor couples chromatin remodeling, checkpoint control, and chromosomal stability. Cancer Cell 2005;7:294–295.
Kohashi K, Izumi T, Oda Y, et al. Infrequent SMARCB1/INI1 gene alteration in epithelioid sarcoma: a useful tool in distinguishing epithelioid sarcoma from malignant rhabdoid tumor. Hum Pathol 2009;40:349–355.
Kohashi K, Oda Y, Yamamoto H, et al. Highly aggressive behavior of malignant rhabdoid tumor: a special reference to SMARCB1/INI1 gene alterations using molecular genetic analysis including quantitative real-time PCR. J Cancer Res Clin Oncol 2007;133:817–824.
Kohashi K, Oda Y, Yamamoto H, et al. SMARCB1/INI1 protein expression in round cell soft tissue sarcomas associated with chromosomal translocations involving EWS: a special reference to SMARCB1/INI1 negative variant extraskeletal myxoid chondrosarcoma. Am J Surg Pathol 2008;32:1168–1174.
Oda Y, Tsuneyoshi M . Extrarenal rhabdoid tumors of soft tissue: clinicopathological and molecular genetic review and distinction from other soft-tissue sarcomas with rhabdoid features. Pathol Int 2006;56:287–295.
Bourdeaut F, Fréneaux P, Thuille B, et al. hSNF5/INI1-deficient tumours and rhabdoid tumours are convergent but not fully overlapping entities. J Pathol 2007;211:323–330.
Cheng JX, Tretiakova M, Gong C, et al. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol 2008;21:647–652.
Hornick JL, Dal Cin P, Fletcher CD . Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 2009;33:542–550.
Hoot AC, Russo P, Judkins AR, et al. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol 2004;28:1485–1491.
Sigauke E, Rakheja D, Maddox DL, et al. Absence of expression of SMARCB1/INI1 in malignant rhabdoid tumors of the central nervous system, kidneys and soft tissue: an immunohistochemical study with implications for diagnosis. Mod Pathol 2006;19:717–725.
Tsuneyoshi M, Daimaru Y, Hashimoto H, et al. The existence of rhabdoid cells in specified soft tissue sarcomas. Histopathological, ultrastructural and immunohistochemical evidence. Virchows Arch A Pathol Anat Histopathol 1987;411:509–514.
Evans HL, Shipley J . Leiomyosarcoma. In: Fletcher CDM, Unni KK, Mertens F (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, France, 2002, pp 131–134.
Fisher C, van den Berg E, Molenaar WM . Adult fibrosarcoma. In: Fletcher CDM, Unni KK, Mertens F (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, France, 2002, pp 100–101.
Guillou L, Kaneko Y . Epithelioid sarcoma. In: Fletcher CDM, Unni KK, Mertens F (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, France, 2002, pp 205–207.
Scheithauer BW, Louis DN, Humter S, et al. Malignant peripheral nerve sheath tumour (MPNST). In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds). World Health Organization Classification of Tumours of the Central Nervous System. IARC Press: Lyon, France, 2007, pp 160–162.
Schofield D . Extrarenal rhabdoid tumour. In: Fletcher CDM, Unni KK, Mertens F (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon, France, 2002, pp 219–220.
Weyers W, Mentzel T, Kasper RC, et al. Fibrohistiocytic and histiocytic tumours. In: LeBoit PE, Burg G, Weedon D, Sarasain A (eds). World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. IARC Press: Lyon, France, 2006, pp 254–262.
Jin L, Majerus J, Oliveira A, et al. Detection of fusion gene transcripts in fresh-frozen and formalin-fixed paraffin-embedded tissue sections of soft-tissue sarcomas after laser capture microdissection and RT-PCR. Diagn Mol Pathol 2003;12:224–230.
Donner LR, Wainwright LM, Zhang F, et al. Mutation of the INI1 gene in composite rhabdoid tumor of the endometrium. Hum Pathol 2007;38:935–939.
Miettinen M, Limon J, Niezabitowski A, et al. Patterns of keratin polypeptides in 110 biphasic, monophasic, and poorly differentiated synovial sarcomas. Virchows Arch 2000;437:275–283.
Folpe AL, Schmidt RA, Chapman D, et al. Poorly differentiated synovial sarcoma: immunohistochemical distinction from primitive neuroectodermal tumors and high-grade malignant peripheral nerve sheath tumors. Am J Surg Pathol 1998;22:673–682.
van de Rijn M, Barr FG, Xiong QB, et al. Poorly differentiated synovial sarcoma: an analysis of clinical, pathologic, and molecular genetic features. Am J Surg Pathol 1999;23:106–112.
de Bruijn DR, dos Santos NR, Thijssen J, et al. The synovial sarcoma associated protein SYT interacts with the acute leukemia associated protein AF10. Oncogene 2001;20:3281–3289.
DiMartino JF, Ayton PM, Chen EH, et al. The AF10 leucine zipper is required for leukemic transformation of myeloid progenitors by MLL-AF10. Blood 2002;99:3780–3785.
Eid JE, Kung AL, Scully R, et al. p300 interacts with the nuclear proto-oncoprotein SYT as part of the active control of cell adhesion. Cell 2000;102:839–848.
Nagai M, Tanaka S, Tsuda M, et al. Analysis of transforming activity of human synovial sarcoma-associated chimeric protein SYT-SSX1 bound to chromatin remodeling factor hBRM/hSNF2 alpha. Proc Natl Acad Sci USA 2001;98:3843–3848.
Thaete C, Brett D, Monaghan P, et al. Functional domains of the SYT and SYT-SSX synovial sarcoma translocation proteins and co-localization with the SNF protein BRM in the nucleus. Hum Mol Genet 1999;8:585–591.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research (B) (no. 21390107) and Young Scientists (B) (no. 21790356) from the Japan Society for the Promotion of Science, Tokyo, Japan. The English used in this paper was revised by KN International (http://www.kninter.com/).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kohashi, K., Oda, Y., Yamamoto, H. et al. Reduced expression of SMARCB1/INI1 protein in synovial sarcoma. Mod Pathol 23, 981–990 (2010). https://doi.org/10.1038/modpathol.2010.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2010.71
Keywords
This article is cited by
-
Molekularpathologie bei ausgewählten Weichgewebssarkomen: diagnostisch und therapeutisch relevante Aberrationen
Die Onkologie (2023)
-
Dedifferentiation-like tubular and solid carcinoma of the stomach shows phenotypic divergence and association with deficient SWI/SNF complex
Virchows Archiv (2022)
-
Soft Tissue Special Issue: Skeletal Muscle Tumors: A Clinicopathological Review
Head and Neck Pathology (2020)
-
The nucleosome acidic patch and H2A ubiquitination underlie mSWI/SNF recruitment in synovial sarcoma
Nature Structural & Molecular Biology (2020)
-
Expression and potential role of SNF5 in endometrial carcinoma
BMC Women's Health (2019)