Abstract
Almost all primary retroperitoneal liposarcomas can be classified as well-/dedifferentiated liposarcoma. Rarely, however, primary retroperitoneal liposarcoma is classified as myxoid/round cell liposarcoma, based on the presence of myxoid areas and vascular crow's feet pattern, which has resulted in a debate on the classification of liposarcoma in the retroperitoneum. Genetically, myxoid/round cell liposarcoma and well-/dedifferentiated liposarcoma are different diseases. Myxoid/round cell liposarcoma is characterized by a translocation causing FUS-CHOP or EWSR1-CHOP fusion, whereas well-/dedifferentiated liposarcoma is characterized by an amplification of the 12q13-15 region, including MDM2 and CDK4 genes. As myxoid/round cell liposarcoma is highly radio- and chemosensitive, differentiation between subtypes is important to optimize treatment. We studied whether primary retroperitoneal liposarcomas diagnosed as myxoid/round cell liposarcoma represent molecularly true myxoid/round cell liposarcoma or are histopathological mimics and represent well-/dedifferentiated liposarcoma. Primary retroperitoneal myxoid/round cell liposarcoma (n=16) were compared to primary extremity myxoid/round cell liposarcoma (n=20). Histopathological and immunohistochemical features were studied. Amplification status of the 12q13-15 region was studied using a multiplex ligation-dependent probe amplification analysis, and FUS-CHOP or EWS-CHOP translocations were studied using RT-PCR. In primary retroperitoneal myxoid/round cell liposarcoma, MDM2 and CDK4 staining was both positive in 12 of 15 cases. In primary extremity myxoid/round cell liposarcoma, MDM2 was negative in 18/20 and CDK4 was negative in all cases. Multiplex ligation-dependent probe amplification showed the amplification of 12q13-15 region in 16/16 primary retroperitoneal myxoid/round cell liposarcomas and in 1/20 primary extremity myxoid/round cell liposarcomas. Translocation was present in all (18/18) primary extremity myxoid/round cell liposarcomas, but absent in all primary retroperitoneal myxoid/round cell liposarcomas. On the basis of immunohistochemical and molecular characteristics, apparent primary retroperitoneal myxoid/round cell liposarcoma can be recognized as well-/dedifferentiated liposarcoma with morphological features mimicking myxoid/round cell liposarcoma. In these cases, treatment should probably be specifically designed as for well-/dedifferentiated liposarcoma. Moreover, finding of myxoid/round cell liposarcoma translocations in a retroperitoneal localization is highly suggestive of metastasis and should prompt search for a primary localization outside the retroperitoneum.
Similar content being viewed by others
Main
Liposarcomas are a rare type of malignancy with a very heterogeneous morphological appearance and clinical course. The therapeutic approach is based on histopathological classification and is dependent on primary localization.
Liposarcoma occurs in four main histopathological classes that are characterized by a distinct morphological spectrum and clear molecular features. The first class of well differentiated liposarcoma includes lipoma-like, sclerosing, inflammatory and spindle cell subtypes. The second class of dedifferentiated liposarcoma occurs in up to 10% of well differentiated liposarcoma of any type and has a more aggressive course. The third class consists of myxoid liposarcoma of which a proportion of cases progresses to round cell liposarcoma, and the last class encompasses the often poorly differentiated pleomorphic liposarcoma.1, 2
The anatomic distribution of liposarcoma appears to be closely related to histological subtype. myxoid/round cell liposarcoma and pleomorphic liposarcoma have a predilection for the extremities, whereas well-/dedifferentiated liposarcomas occur predominantly in the retroperitoneum.
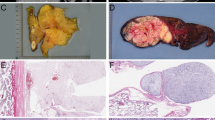
In retroperitoneal tumors, apart from the more generally found morphological features of lipoma-like, sclerosing or dedifferentiated liposarcoma, also components resembling myxoid/round cell liposarcoma are incidentally found. On the basis of histological appearance with myxoid areas and vascular crow's feet pattern (Figure 1), 5% of primary retroperitoneal liposarcomas are reported as myxoid or round cell liposarcoma.3
From a molecular point of view, myxoid/round cell liposarcoma and well-/dedifferentiated liposarcoma are different entities. In more than 95% of myxoid/round cell liposarcoma, a classical t(12;16) (q13;p11) or t(12;22) (q13;q12) translocation can be found, with a FUS-CHOP or EWSR1-CHOP gene fusion, respectively. Characteristic genetic alteration for well-/dedifferentiated liposarcoma is the amplification of the 12q13-15 region, which, among many other genes, includes MDM2 and CDK4.4, 5, 6, 7 Both alterations seem to be mutually exclusive and distinctive for their disease classes.
Myxoid/round cell liposarcoma is highly radio- and chemosensitive,8, 9, 10 and differentiation between the subtypes of liposarcoma is important to indicate prognosis, and especially to tailor treatment. Myxoid/round cell liposarcoma has several mimics, including myxoid chondrosarcoma, myxoid malignant fibrous histiocytoma (myxofibrosarcoma), and in selected situations, also well-/dedifferentiated liposarcoma. The latter problem is mostly encountered when edematous and degenerative changes are present and has specifically resulted in a debate between specialists on the classification of liposarcoma in the retroperitoneum and confusion among the interested pathologists.
We studied whether primary retroperitoneal liposarcomas diagnosed as myxoid/round cell liposarcoma represent molecularly true myxoid/round cell liposarcoma or are rather histopathological mimics, and actually represent well-/dedifferentiated liposarcoma with myxoid-like changes.
Materials and methods
Patient Selection
From all primary retroperitoneal liposarcomas diagnosed in The Netherlands Cancer Institute between 1977 and 2006 (n=68), 16 patients were classified as myxoid/round cell liposarcoma. All cases were reviewed according to the criteria of the most recent WHO classification, and the presence of >80% of myxoid areas and micro crow's feet vasculature was confirmed.11 Cases were considered as primary retroperitoneal lesions in case of retroperitoneal soft tissue monolocalization with or without the evidence of pulmonary metastasis. In case of one or more additional synchronous soft tissue localizations, retroperitoneal liposarcoma was considered only primary, if this would be the dominant lesion. No such cases, however, were identified in this series. At least 10 tumor blocks were reviewed per tumor. As a control group, 20 cases of myxoid/round cell liposarcoma with a primary localization in the extremities, and diagnosed in the same period, were selected. Detailed patient and tumor characteristics of both groups are shown in Table 1.
Immunohistochemical Analysis of MDM2 and CDK4
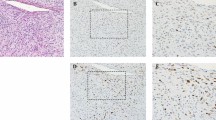
Tissue microarrays were constructed according to standard methods using triplicate 1 mm cores of representative areas of each tumor. Appropriate coordinates and staining controls were included. Immunohistochemical staining for MDM2 and CDK4 was performed according to standard methods. In brief, after a pretreatment of citrate-based microwave antigen retrieval, the sections were incubated with the following antibodies overnight at 4°C: anti-MDM2 antibody (dilution 1/3000; BD Biosciences, Erembodegem, Belgium) and anti-CDK4 antibody (dilution 1/1500; Thermo Fisher Scientific, Fremont, CA, USA) and visualized with diaminobenzidine. The percentage of tumor cells with nuclear staining was assessed semiquantitatively and averaged for the triplet cores per patient. Staining intensity was ranked in four levels (Tables 2 and 3 and Figure 2). Immunohistochemical stainings on tissue microarray were scored by two observers (DdJ and RdV). In cases of discrepancies or equivocal interpretations, consensus was obtained at the multiheaded microscope. If needed, immunohistochemistry was performed on full sections of the tumor and compared with TMA results.
Detection of FUS-CHOP and EWSR1-CHOP Fusion Gene
RNA was extracted from representative sections of the formalin-fixed, paraffin-embedded specimens according to the manufacturer's method using high pure RNA paraffin kit (Roche, Basel, Switzerland).12 Microdissection to optimize tumor cell content was performed, if necessary. RNA concentration was measured using a nanodrop spectrophotometer. RT-PCR amplification was performed using one-step RT-PCR. Because of the limiting quality of RNA from formalin-fixed, paraffin-embedded material, different breakpoints were detected, using a combination of FUS-CHOP and EWSR1-CHOP-specific internal primers, resulting in small PCR products (Table 4). PCR cycles were operated on a regimen of 30 s of denaturation at 95°C, 30 s of primer annealing at 68°C, and 30 s extension/synthesis at 72°C for 30 cycles. PCR products were separated by electrophoresis on 2.5% agarose gels containing ethidium bromide, visualized by UV light and photographed. To confirm the type of fusion gene, PCR products were compared with positive controls of the different fusion gene combinations as confirmed by sequencing.
Molecular Analysis by Multiplex Ligation-Dependant Probe Amplification
The recently described multiplex ligation-dependant probe amplification technique13 is a high throughput, PCR-based, method to determine the relative copy number of various DNA sequences in small samples of human DNA. It is based on the annealing of a mixture of hemiprobes on their cognate DNA sequences. One of the hemiprobes contains stuffer DNA of variable length (19–370 bp), which allows multiplex detection using capillary sequencer.
The SALSA- multiplex ligation-dependant probe amplification kits, P007 and P172 tests, were developed and manufactured by MRC-Holland. The preparation and sequences of the probes have been described elsewhere.13 In short, 200 ng target DNA/5 μl of 10 mM Tris (pH8)-0,1 mM EDTA was denaturated for 5 min at 98°C after which 3 μl of the probe mix was added. The mixture was heated at 95°C for 1 min and incubated at 60°C overnight (16 h). Ligation was performed with the temperature-stable ligase-65 enzyme (MRC-Holland) for 15 min at 54°C. Then, the ligase was inactivated by incubation for 5 min at 98°C. In all, 10 μl of this ligation mix was premixed with 30 μl of PCR buffer and put in a PCR machine at 60°C. Subsequently, a 10 μl mix was added containing deoxynucleoside triphosphate, Taq polymerase, and one unlabeled and one carboxyfluoriscein-labeled PCR primer, which are complementary to the universal primer sequences. PCR was carried out for 35 cycles (30 s at 95°C, 30 s at 60°C, and 60 s at 72°C). The fragments were analyzed on an ABI model 310 or 3700 capillary sequencer (Applied Biosystems) using Genescan-ROX 500 size standards (Applied Biosystems). Fragment analysis was performed using Genescan and Genotyper software, amplifications or deletions were calculated and evaluated, compared to 15 samples DNA from normal tissues. After normalization, each probe set was scored as 0.01–1.00 deleted, 1.01–2.99 normal copy number, 3.00–3.99 copy number gain, and finally ≥4.00 amplification.
Results
Detailed clinical and tumor features of both groups are shown in Table 1. Sixteen patients (seven female and nine male) were diagnosed with primary retroperitoneal myxoid/round cell liposarcoma and 20 (eight female and 12 male) with primary extremity myxoid/round cell liposarcoma.
Median tumor diameter at histology was 23 cm (13–35) in the retroperitoneal group and 14 cm (4–30) in the extremity group. Most tumors were intermediate grade (n=24), the remaining sarcomas were high grade (n=12). Most extremity tumors were resected, followed by radiotherapy indicated by often inevitable narrow margins of resection. Radiation therapy in retroperitoneal tumors has been applied in this study period only in selected cases.
Immunohistochemical Analysis of MDM2 And CDK4
Detailed immunohistochemical results and examples can be found in Tables 2 and 3 and Figure 2. Immunohistochemical analysis was interpretable in 35 of 36 (97%) cases. Semiquantitative analysis of primary retroperitoneal myxoid/round cell liposarcoma patients by immunohistochemical detection of MDM2 staining showed in 12 of 15 (80%) patients a well-defined positive staining, whereas one patient showed weak staining and two patients (13%) showed a very weak staining. Positive staining of CDK4 was seen in 12 of 15 (80%) patients; two (13%) patients showed weak staining and one was negative in the retroperitoneal group.
In the primary extremity myxoid/round cell liposarcoma group, 18 of 20 (90%) patients were negative for MDM2, and two cases (10%) showed weak staining. All primary extremity myxoid/round cell liposarcoma cases were negative for immunohistochemical staining of CDK4.
Detection of FUS-CHOP and EWSR1-CHOP Fusion Gene
No FUS-CHOP and EWSR1-CHOP translocations were found in any primary retroperitoneal myxoid/round cell liposarcoma. In 18 of 20 extremity myxoid/round cell liposarcoma, a FUS-CHOP translocation was found. In two cases, extracted RNA was of insufficient quality, precluding analysis.
Amplification of 12q13-15 by Multiplex Ligation-Dependant Probe Amplification Analysis
All patients in both groups could be studied with molecular multiplex ligation-dependant probe amplification analysis; see Supplementary data, Table 3 and Figure 3. Both the multiplex ligation-dependant probe amplification kits, which were used, were interpreted after calculation of visualized peak surface area on an electropherogram,13 and normalized to the results of 15 samples' normal reference tissues, assuming two copies for each target probe. Amplification was defined as four target sequence copies or more.
(a and b) Multiplex ligation-dependant probe amplification results of 79 marker genes in 16 retroperitoneal lesions show amplification of region 12q13-15. (c and d) Multiplex ligation-dependant probe amplification results of 79 marker genes in 20 extremity lesions. The threshold level of amplification is set at 4, the region of normal diploid copy numbers is highlighted in gray.
In all, 5 of the 79 probes targeted the 12q13-15 region, a single IFNG probe and two different probes on the CDK4 and MDM2 gene. Amplification of the CDK4 gene was found in 15 of 16 (94%) and amplification of the MDM2 gene was found in 16 of 16 (100%) primary retroperitoneal myxoid/round cell liposarcoma patients, and the amplification of the IFNG gene was found in five cases (31%). Although the IFNG gene is supposed to be situated in between CDK4 and MDM2, these results, which could implicate two different amplicons, are in accordance with the results reported by others.14, 15, 16
Multiplex ligation-dependant probe amplification of the primary extremity myxoid/round cell liposarcoma group showed, in 1 of 20 (5%) patients, an amplification (4.38) in one of the five probes targeting the 12q13-15 region.
Chromosomal Gains And Losses of Other Regions As Detected with Multiplex Ligation-Dependant Probe Amplification
Using multiplex ligation-dependant probe amplification, 74 additional chromosomal regions were screened for amplification. In the primary retroperitoneal myxoid/round cell liposarcoma group, additional amplifications of the TNFRNF1B gene located on chromosome 1p36.3 was found in four patients; the TERT gene located on chromosome 5p25 showed amplification in five patients; the MYC gene located on chromosome 8q24 showed amplification in two patients, and CCND2 on 12p13 showed amplification in three patients. Furthermore, single amplifications of SLA, PTK2, TINF2, NFKBIA, SCYA3, B2M, MOS, GSTP1, PTPN1, AURKA, BIRC4, and TFF1 genes were found. Some of these changes have been correlated to lipomatous tumors by others previously.17
Deletions were found in 40 of 1264 samples (ranged 0.56–0.1.00) of primary retroperitoneal myxoid/round cell liposarcoma patients and in 10 of 1580 samples (ranged 0.22–0.98) of primary extremity myxoid/round cell liposarcoma patients.
By using a Pearson test, positive correlations were found comparing staining intensities of CDK4 and MDM2 to multiplex ligation-dependant probe amplification results (CDK4: +0,420, p0,021; MDM2: +0,386, p0,035), which can be seen in Table 3 where the level of staining in a majority of cases reflects the level of DNA copy number of MDM2 and CDK4 found by multiplex ligation-dependant probe amplification.
Discussion
This study shows that cases of primary retroperitoneal liposarcoma with morphological features suggestive of myxoid/round cell liposarcoma actually represent well-/dedifferentiated liposarcoma on the basis of immunohistochemical and molecular features. Most importantly, the myxoid/round cell liposarcoma-defining and characteristic FUS-CHOP or EWSR1-CHOP translocation was not found, but amplification of 12q13-15 including MDM2 and CDK4 was found in all cases. Therefore, this study shows that the histomorphological appearance does not always and necessarily reflect the genetic basis.
The classification difficulties are mostly encountered when edematous and degenerative changes are present which has specifically resulted in a debate on the classification of liposarcoma in the retroperitoneum between specialists and confusion among the interested pathologists. Most importantly, the clinical relevance of the distinction of both subtypes of liposarcoma lies in the fact that myxoid/round cell liposarcoma is highly radio- and chemosensitive.8, 9, 10 Therefore, differentiation between subtypes of liposarcoma is important to indicate the prognosis and choose the appropriate treatment. Surgery is the standard therapy for sarcoma, including liposarcoma. Resection of retroperitoneal liposarcomas continues to be a challenge for the surgeon, in particular given their large size and often central location with involvement of vital structures and organs. Histological grade of a retroperitoneal (lipo) sarcoma and its complete resection remain the most important predictors for local recurrence and overall survival.3, 18, 19 As in our institute, intermediate or high-grade sarcomas are, if feasible, treated by surgery combined with (neo)adjuvant radiotherapy, whereas low-grade well-/dedifferentiated liposarcomas are treated by resection only.20
Chemotherapy sensitivity varies considerably between liposarcoma subtypes, with a significant higher response rate to the first-line chemotherapy in myxoid/round cell liposarcoma as compared to well-/dedifferentiated liposarcoma (48 vs 11%).10 These reports are emphasizing the need for adequate staging and classification of the liposarcoma subtype to choose the most appropriate treatment. In primary retroperitoneal myxoid/round cell liposarcoma, immunohistochemical or molecular analysis on (biopsy) specimens can support or reject the morphologic classification, and should therefore be considered in case of doubt.
Now, the existence of true primary myxoid/round cell liposarcoma in the retroperitoneum can be strongly doubted. Reports of genetically proven classic myxoid/round cell liposarcoma in the retroperitoneum should be interpreted in a different way.9, 21, 22, 23 All reported myxoid/round cell liposarcoma patients with retroperitoneal localizations showed multiple localizations, of which the retroperitoneum was the second or latter localization. Indeed, in our and in other series, the retroperitoneum is one of the most common sites of second localization of myxoid/round cell liposarcoma.24 Therefore, the importance of searching for a primary tumor must be emphasized, when diagnosing a classic myxoid/round cell liposarcoma in the retroperitoneum on molecular biologic grounds as the first presentation of malignancy.
As in approximately 95% of myxoid/round cell liposarcoma cases, a specific FUS-CHOP or EWSR1-CHOP translocation can be found; a negative translocation analysis does not always exclude myxoid/round cell liposarcoma. To positively establish the well-/dedifferentiated liposarcoma character of a primary retroperitoneal liposarcoma, various methods can be used. Here, we used multiplex ligation-dependant probe amplification combined with immunohistochemical analysis, which can lead to an undisputable diagnosis, showing that the combination of diagnostic tools can be useful to unravel its biologic basis. PCR-based assays, either custom made and specifically targeted to the 12q region or a broader targeted commercially available, such as multiplex ligation-dependant probe amplification may be appropriate. Our study shows that PCR-based methods, such as multiplex ligation-dependant probe amplification was evaluable in all tested samples and may be more sensitive than immunohistochemistry for MDM2 and/or CDK4, but is relatively costly and labor intense, and may be reserved for ambiguous cases in daily practice. Alternatively, the amplification status of the 12q13-15 region can be assessed using fluorescence in situ hybridization techniques, which have also been shown to be a highly sensitive and specific method, but is sometimes very difficult to interpret in cases with necrosis, low tumor cell percentages or poor tissue preservation.
Conclusion
This study shows that myxoid/round cell liposarcoma histopathologic appearance in primary retroperitoneal liposarcoma can be explained by the presence of focal myxoid-like changes in well-/dedifferentiated liposarcoma and that the multiple diagnostic tests on primary retroperitoneal liposarcomas can corroborate to a specific diagnosis on which an appropriate treatment can be based. In addition, finding of myxoid/round cell liposarcoma translocations in a retroperitoneal localization is highly suggestive of metastasis and should prompt the search for a primary localization outside the retroperitoneum.25, 26
References
Kindblom LG . Lipomatous tumors-how we have reached our present views, what controversies remain and why we still face diagnostic problems: a tribute to Dr Franz Enzinger. Adv Anat Pathol 2006;13:279–285.
Downes KA, Goldblum JR, Montgomery EA, et al. Pleomorphic liposarcoma: a clinicopathologic analysis of 19 cases. Mod Pathol 2001;14:179–184.
Singer S, Antonescu CR, Riedel E, et al. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg 2003;238:358–370.
Singer S, Socci ND, Ambrosini G, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer Res 2007;67:6626–6636.
Dei Tos AP, Doglioni C, Piccinin S, et al. Coordinated expression and amplification of the MDM2, CDK4, and HMGI-C genes in atypical lipomatous tumours. J Pathol 2000;190:531–536.
Hostein I, Pelmus M, Aurias A, et al. Evaluation of MDM2 and CDK4 amplification by real-time PCR on paraffin wax-embedded material: a potential tool for the diagnosis of atypical lipomatous tumours/well-differentiated liposarcomas. J Pathol 2004;202:95–102.
Italiano A, Cardot N, Dupre F, et al. Gains and complex rearrangements of the 12q13-15 chromosomal region in ordinary lipomas: the ‘missing link’ between lipomas and liposarcomas? Int J Cancer 2007;121:308–315.
Pitson G, Robinson P, Wilke D, et al. Radiation response: an additional unique signature of myxoid liposarcoma. Int J Radiat Oncol Biol Phys 2004;60:522–526.
Engstrom K, Bergh P, Cederlund CG, et al. Irradiation of myxoid/round cell liposarcoma induces volume reduction and lipoma-like morphology. Acta Oncol 2007;46:838–845.
Jones RL, Fisher C, Al-Muderis O, et al. Differential sensitivity of liposarcoma subtypes to chemotherapy. Eur J Cancer 2005;41:2853–2860.
Fletcher CD . Pathology and Genetics Tumours of Soft Tissue and Bone, WHO classification book for tumours of soft tissue and bone, 2002.
Vogelstein B, Gillespie D . Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci USA 1979;76:615–619.
Schouten JP, McElgunn CJ, Waaijer R, et al. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 2002;30:e57.
Italiano A, Bianchini L, Keslair F, et al. HMGA2 is the partner of MDM2 in well-differentiated and dedifferentiated liposarcomas whereas CDK4 belongs to a distinct inconsistent amplicon. Int J Cancer 2008;122:2233–2241.
Reifenberger G, Ichimura K, Reifenberger J, et al. Refined mapping of 12q13-q15 amplicons in human malignant gliomas suggests CDK4/SAS and MDM2 as independent amplification targets. Cancer Res 1996;56:5141–5145.
Simon R, Struckmann K, Schraml P, et al. Amplification pattern of 12q13-q15 genes (MDM2, CDK4, GLI) in urinary bladder cancer. Oncogene 2002;21:2476–2483.
Hameed M . Pathology and genetics of adipocytic tumors. Cytogenet Genome Res 2007;118:138–147.
Singer S, Corson JM, Demetri GD, et al. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann Surg 1995;221:185–195.
Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg 1998;228:355–365.
Mollabashy A, Virkus WW, Zlotecki RA, et al. Radiation therapy for low-grade soft tissue sarcoma. Clin Orthop Relat Res 2002;397:190–195.
Antonescu CR, Elahi A, Healey JH, et al. Monoclonality of multifocal myxoid liposarcoma: confirmation by analysis of TLS-CHOP or EWS-CHOP rearrangements. Clin Cancer Res 2000;6:2788–2793.
Estourgie SH, Nielsen GP, Ott MJ . Metastatic patterns of extremity myxoid liposarcoma and their outcome. J Surg Oncol 2002;80:89–93.
Antonescu CR, Elahi A, Humphrey M, et al. Specificity of TLS-CHOP rearrangement for classic myxoid/round cell liposarcoma: absence in predominantly myxoid well-differentiated liposarcomas. J Mol Diagn 2000;2:132–138.
Pearlstone DB, Pisters PW, Bold RJ, et al. Patterns of recurrence in extremity liposarcoma: implications for staging and follow-up. Cancer 1999;85:85–92.
Sirvent N, Coindre JM, Maire G, et al. Detection of MDM2-CDK4 amplification by fluorescence in situ hybridization in 200 paraffin-embedded tumor samples: utility in diagnosing adipocytic lesions and comparison with immunohistochemistry and real-time PCR. Am J Surg Pathol 2007;31:1476–1489.
Shimada S, Ishizawa T, Ishizawa K, et al. The value of MDM2 and CDK4 amplification levels using real-time polymerase chain reaction for the differential diagnosis of liposarcomas and their histologic mimickers. Hum Pathol 2006;37:1123–1129.
Acknowledgements
This study is dedicated to the memory of our colleague JL (Hans) Peterse, pathologist, who tragically died suddenly during this project. We thank D Veldhuizen, L Boerrigter, and A Ariaens for expert technical support. We also thank L Frenken MD for his dedicated work in data management.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/conflict of interest
No conflict of interest and sources of support in connection with this work are present.
Supplementary Information accompanies the paper on Modern Pathology website (http://www.nature.com/modpathol)
Supplementary information
Rights and permissions
About this article
Cite this article
de Vreeze, R., de Jong, D., Tielen, I. et al. Primary retroperitoneal myxoid/round cell liposarcoma is a nonexisting disease: an immunohistochemical and molecular biological analysis. Mod Pathol 22, 223–231 (2009). https://doi.org/10.1038/modpathol.2008.164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.2008.164
Keywords
This article is cited by
-
Perinephric myxoid pseudotumor of fat: a multimodality imaging case series
Abdominal Radiology (2022)
-
Oncologic outcomes of pre- versus post-operative radiation in Resectable soft tissue sarcoma: a systematic review and meta-analysis
Radiation Oncology (2020)
-
Coexistence of gastric gastrointestinal stromal tumor, intro-abdominal and retroperitoneal liposarcomas –a case report
BMC Cancer (2018)
-
Imaging appearance of well-differentiated liposarcomas with myxoid stroma
Skeletal Radiology (2018)
-
Liposarcomas of the posterior mediastinum: clinicopathologic study of 18 cases
Modern Pathology (2015)