Abstract
As CD4+CD25+Foxp3+ regulatory T cells (Tregs) play crucial immunomodulatory roles during infections, one key question is how these cells are controlled during antimicrobial immune responses. Mechanisms controlling their homeostasis are central to ensure efficient protection against pathogens, as well as to control infection-associated immunopathology. Here we studied how their viability is regulated in the context of mouse oropharyngeal candidiasis (OPC) infection, and found that these cells show increased protection from apoptosis during late phase of infection and reinfection. Tregs underwent reduced cell death because they are refractory to T cell receptor restimulation-induced cell death (RICD). We confirmed their resistance to RICD, using mouse and human Tregs in vitro, and by inducing α-CD3 antibody-mediated apoptosis in vivo. The enhanced viability is dependent on increased transforming growth factor-β1 (TGF-β1) signaling that results in upregulation of cFLIP (cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein) in Tregs. Protection from cell death is abrogated in the absence of TGF-β1 signaling in Tregs during OPC infection. Taken together, our data unravel the previously unrecognized role of TGF-β1 in promoting Treg viability, coinciding with the pronounced immunomodulatory role of these cells during later phase of OPC infection, and possibly other mucosal infections.
Similar content being viewed by others
INTRODUCTION
CD4+CD25+Foxp3+ regulatory T cells (Tregs) are central for dominant tolerance to self-antigens and innocuous commensals.1, 2, 3 A majority of Tregs are natural or thymic Tregs, but a few also arise in vivo in the periphery from conventional naive CD4 T cells or can be generated in vitro. Reduced frequency of circulating Tregs and their defective functions are associated with autoimmunity and inflammation.4 In contrast, in patients with cancer, Treg enrichment at the tumor sites is implicated in regulating tumor immune-surveillance.5 As the size of the Treg population determines disease outcomes, manipulating it is an exciting strategy in human immune therapy. Therefore, basic mechanisms of Treg viability and proliferation in different niches are being explored.6, 7 With respect to lymphocyte apoptosis, restimulation-induced cell death (RICD)8, 9 and cytokine withdrawal-induced death10 govern effector cell homeostasis in vivo.11 RICD, also referred to as activation-induced cell death,12, 13 is responsible for the attrition and homeostasis of expanded T-cell population after antigen clearance.9, 14 RICD is an extrinsic apoptosis mechanism that is signaled through Fas and tumor necrosis factor-α receptors, leading to activation of caspases.15 Cytokine withdrawal-induced death is an intrinsic mitochondrial apoptosis that occurs because of cellular stressors such as withdrawal of survival cytokines,16 involving the activation of Bim and caspases.10, 17, 18 Interleukin-2 (IL-2) and other γ-chain cytokines control Treg survival, without which Tregs are susceptible to Bim-dependent cytokine withdrawal-induced death.19, 20 It is well known that along with Tregs, RICD8, 9, 21 and cytokine withdrawal-induced death are also10, 16 required for immune homeostasis following antigen clearance during infections.22, 23 Although Bim and transforming growth factor-β1 (TGF-β1) have been implicated in regulating the size of Tregs,24, 25 their role in Treg RICD is unknown. Two in vitro studies have examined RICD in Tregs, but the mechanistic details and the physiological relevance were not explored.26, 27 Although Foxp3+ Tregs are shown to accumulate at infection sites, little is known about their apoptosis mechanisms and homeostasis during infections.
In the context of microbial infections, Tregs perform important roles to limit inflammation, especially at late time points of infection. We and others have previously reported their immune-protective functions that vary depending on the context of infections.28, 29, 30, 31 Here we have systematically shown that Tregs resist RICD compared with T effector cells during oropharyngeal candidiasis (OPC) infection and chronic lymphocytic choriomeningitis virus (LCMV) infection. Increased survival is dependent on TGF-β1 signaling and upregulation of cFLIP (cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein) in Tregs. This novel mechanism that offers a survival advantage to these critical immunomodulatory T cells may be important for immune homeostasis and resolution of immunopathology after infection clearance, and possibly other inflammatory conditions.
RESULTS
Tregs undergo reduced apoptosis during later phases of oral Candida infection and reinfection in vivo
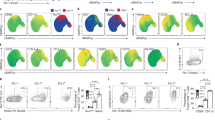
As we have previously shown that Tregs play important roles in oral candidiasis model,30 we first sought examine the Treg frequency during the course of OPC infection. We infected Foxp3GFP reporter mice with Candida, isolated the cells from draining cervical lymph nodes (CLNs), and estimated the frequency of Foxp3GFP+ cells using flow cytometric analyses. As expected, sham-infected mice showed ∼10% of Foxp3GFP+ cells among CD4+ T cells (Figure 1a,b). Although on days 1 and 2 there was a drop in the frequency of GFP+ Tregs, the frequency of Tregs kept increasing even 5 days after infection in the CLNs (Figure 1a,b). We have previously shown that mice clear the infection in ∼3–4 days, and in the absence of Tregs they succumb to severe infection and immunopathology.30, 31 Although tumor necrosis factor-α has protective functions during acute infection,32 Tregs reduce tumor necrosis factor-α levels in CD4 T cells, thereby limiting tongue inflammation at later time points. Therefore, we hypothesized that Tregs may proliferate or survive better over effector cells at later time points, coinciding with immunopathology control. Although Foxp3+Tregs did not proliferate better than Foxp3GFP− T effector cells, they showed increased viability, as measured by propidium iodide (PI) staining (Supplementary Figure S1A online). Fas- and Bim-dependent apoptosis of effector cells,23, 33 along with immunomodulation by Tregs, are known to contribute to immune response shutdown and control of immunopathology after infections.14, 28 Because Tregs are already known to be susceptible to Bim-dependent apoptosis induced by cytokine withdrawal,7, 19 we hypothesized that their increased viability is attributed to their reduced ability to undergo RICD during infection. Therefore, we induced RICD of T cells in vivo by reinfecting Foxp3GFP mice at late time points of primary Candida infection. We assessed the viability of the cells on day 1 after reinfection. We harvested the cells from axillary lymph nodes and CLN, the draining lymph nodes, as well as spleen and inguinal lymph nodes to assess CD4+ cell viability. We refer to the non-Treg (Foxp3−) cells activated by the infection in vivo as effector cells (Teffs). We gated on the control CD4+Foxp3GFP− Teffs and CD4+ CD25+Foxp3GFP+ Tregs (Supplementary Figure S1B, C), and measured the viability by PI staining. We found that the frequency of PI+ dead cells among CD4+Foxp3GFP+ Tregs was 10–12%, and was significantly lower than in CD4+ Foxp3GFP− effector cells (∼24%) in draining lymph nodes (Figure 1c and Supplementary Figure S1C). In addition, by examining the absolute cell numbers ex vivo at various time points after primary infection, we found that although the effector cells undergo an expansion followed by contraction at late time points, Tregs did not show reduction in cell counts (Supplementary Figure S1D) coinciding to increased survival at later time points. In spleen and inguinal lymph nodes, although Tregs had slightly increased viability than effector cells, the differences were smaller than in draining lymph nodes (Figure 1c). Next, we adoptively transferred fluorescence-activated cell sorting (FACS)-sorted naive CD4+CD25−GFP− cells (conventional or control CD4+ cells; Tcons) or CD4+CD25+GFP+ Tregs into Rag1−/− mice, 4 days before primary OPC infection, and assessed their viability 1 day after reinfection. We refer to the non-Treg (Foxp3− Tcons) cells activated by the infection in vivo as Teffs. We found that the frequency of PI+ cells was greater among Teffs than Tregs (Figure 1d), showing that Tregs survive better than conventional CD4 T cells during RICD at late phase of infection. To confirm the role of Fas in the contraction of CD4+ T cells, we infected Fas mutant lympho-proliferation (lpr) mice, comparing with the wild-type (WT) mice. Although there was an increase in the frequency of Tregs in WT mice, there was no increase in lpr mice at late time points (Supplementary Figure S2). These results demonstrate that Fas is largely contributing to contraction of effector cells, without which the apparent increase in proportion of Tregs is not observed at late time points.
Regulatory T cells (Tregs) show increased viability during Candida reinfection and in vivo. (a, b) CD4+Foxp3+ Tregs show increased enrichment during the course of oropharyngeal candidiasis (OPC) in mice. Foxp3GFP reporter mice (n=5/group) were infected with Candida albicans (Candida) as described in Methods. Flow cytometric plots (a) and statistical analyses (b) showing the frequencies of cells expressing CD4 and intracellular Foxp3 (gated on CD4 cells) in cervical lymph nodes (CLNs) at various days (d) after C. albicans infection. Percentages of Foxp3-expressing cells are shown. P-value is determined by Mann–Whitney test. (c) Mice were infected and reinfected as in a. Axillary lymph nodes (ALNs), CLNs, spleen (SPLN), and inguinal lymph nodes (ILNs) were harvested on day 1 after reinfection to examine cell death of Foxp3GFP− Teff (left) and Foxp3GFP+Tregs (right) ex vivo by green fluorescent protein (GFP) and propidium iodide (PI) staining. Flow cytometric contour plots (left panel) and statistical data (right panel) show the frequencies of PI+ cells (gated on CD4 cells). Statistical significance was determined using Mann–Whitney test. (d) Rag1−/− CD45.1 mice (n=5/group) were reconstituted with CD4+Foxp3GFP− Teff or CD4+Foxp3GFP+Treg cells obtained from congenic CD45.2 mice. Recipient mice in each group were reinfected with Candida. ALNs and CLNs were harvested on day 1 after reinfection for examining cell death ex vivo by PI staining (gated on CD45.2+ cells). (e, f) Foxp3GFP reporter mice were injected with phosphate-buffered saline (PBS) or α-CD3 antibody. Flow contour plots of yellow fluorescent protein (YFP) and PI staining histograms (e) of Foxp3YFP− Teff (top) and Foxp3YFP+ Tregs (bottom) and quantification of % PI+ cells (f) of Foxp3GFP− Teff (blue circles) and Foxp3YFP+ Tregs (red squares) 24 h after α-CD3 antibody injection in mice (n=4/group) (gated on CD4+ cells). Statistical significance was determined using Mann–Whitney test. Data from one of three to five independent experiments are shown.
To further validate that Tregs have decreased susceptibility to T cell receptor (TCR)-mediated RICD in vivo, we directly examined whether Tregs resist α-CD3 antibody-mediated deletion in vivo. We injected 100 μg of α-CD3 antibody in Foxp3YFP mice and measured the viability of Tregs and conventional CD4+ cells.34 Correlating to the results from OPC infection experiments, we found that the percentage of PI+ cells was significantly lower among Foxp3YFP+ Tregs compared with Foxp3YFP− cells in vivo in CLN (Figure 1e), spleen, and other lymph nodes upon α-CD3 antibody injection (Figure 1e,f). To confirm whether Tregs resist apoptosis during a chronic infection, we infected the mice with LCMV clone 13, and assessed apoptosis of CD4+ T cells at different time points after infection. We found that at all time points, Tregs showed significantly reduced apoptosis than effector cells after infection in vivo (Supplementary Figure S3).
Mouse and human Tregs are resistant to RICD and show concomitant reduction in active caspase-3 levels
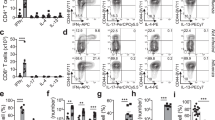
To investigate the mechanism by which Tregs resist RICD, we examined their viability during RICD apoptosis in vitro. We isolated CD4+ CD25+ Foxp3GFP+ cells (Treg) and conventional or control CD4+ CD25− Foxp3GFP− naive T cells (Tcon) from Foxp3GFP reporter mice (Supplementary Figure S4) and stimulated and restimulated them with α-CD3 antibody (1 μg ml−1) and IL-2. We measured the cell viability by their forward scatter shift and PI staining 12–18 h after RICD induction using flow cytometry. Whereas 80–85% of the Tcons were PI+, only 30–40% of Tregs showed PI staining (Figure 2a). Then, we induced RICD with different concentrations of α-CD3 antibody. We measured apoptosis by annexin-V and PI staining (Figure 2b) and found that Tregs underwent significantly reduced apoptosis. Next, we isolated CD4+CD25+CD127lo Tregs and CD4+ CD25− Tcons from human peripheral blood mononuclear cells and induced RICD. Human Tregs also showed resistance to RICD compared with Tcons (Figure 2c,d). Upon induction of RICD, both mouse and human Tregs showed substantially lower levels of cleaved caspase-3 compared with Tcons (Figure 2e,f). Taken together, these results reveal that although conventional CD4 cells are highly susceptible to RICD, Tregs show reduced RICD with concomitantly lower levels of active caspase-3.
Mouse and human regulatory T cells (Tregs) are resistant to restimulation-induced cell death (RICD) and show decreased activated caspase levels. (a) Flow cytometric histogram plots of propidium iodide (PI) staining of mouse Tcons (upper panel) and mouse Tregs (lower panel) derived and pooled from spleen/lymph nodes (SPLN/LN), stimulated under RICD conditions for 20 h with 0 μg ml−1 (left) or 1 μg ml−1 (right) of α-CD3 antibody. As controls, we stimulated the cells with interleukin-2 (IL-2) only, but no α-CD3 antibody (0 μg ml−1). (b) Quantification of % apoptosis of the Tcons (gray bars) or Tregs (black bars) restimulated with indicated concentrations of α-CD3 antibody for 18 h, based on evaluating the annexin-V and PI staining by flow cytometry. (c) Flow cytometric analyses of PI staining of human Tcons (upper panel) and human Tregs (lower panel) stimulated under RICD conditions for 21 h with 0 μg ml−1 (left), 0.5 μg ml−1 (middle), or 1 μg ml−1 (right) of α-CD3 antibody. (d) % Apoptosis quantification of human cells stimulated as in c. (e) Mouse cells or (f) human cells were stimulated for 15 h as in a and c, respectively, and cleaved caspase-3 levels were determined by intracellular staining and flow cytometry. P-values are determined by Mann–Whitney test in b and d. Data in a and b represent at least 10 independent experiments and in c–f are from one of three independent experiments showing similar results.
RICD resistance is not because of retarded proliferation in Tregs
Previous studies show that T cells producing high levels of IL-2 and proliferating at higher rates have an increased propensity to die of RICD.35 Tregs produce little or no IL-2 and proliferate slower that might contribute to their reduced apoptosis. IL-2 was ruled out because we added excess of IL-2 during RICD. However, during the initial stimulation for 4 days, Tregs displayed reduced proliferation compared with Tcons as determined by CPD670 labeling (Figure 3a). We assessed cell death, gating on proliferating and nonproliferating cells, upon RICD. Nonproliferating cells (in the prior stimulation) in both the populations showed little or no apoptosis (Figure 3b,d). However, proliferating Tregs were still exquisitely resistant to RICD compared with proliferating Tcons (Figure 3c,d). Moreover, 16 h after RICD induction, the time point at which we measured apoptosis, there was no difference in proliferation between Tcons and Tregs (data not shown). Moreover, to examine whether Tregs and Tcons are activated at different levels, we tested their nuclear factor (NF)-κB activation by its nuclear translocation. Confocal microscopy revealed that nuclear translocation of the p65 subunit of NF-κB was comparable in Tregs and Tcons after RICD stimulation (Figure 3e). These results showed that RICD resistance of Tregs is not due to their reduced proliferation or activation.
Reduced restimulation-induced cell death (RICD) in regulatory T cells (Tregs) is not dependent on retarded proliferation or reduced activation. (a) Flow cytometric histogram plots showing CPD670 dilution of mouse Tcons (upper panel) and mouse Tregs (lower panel) derived and pooled from spleen/lymph nodes (SPLN/LN), sorted as described in Methods, and stimulated for 4 days with α- CD3, α-CD28, and interleukin-2 (IL-2) without RICD induction. CPD670 dilution shows the frequency of proliferating (prol.) and nonproliferating cells (n.prol.). (b, c) Tcon or Treg cells labeled and stimulated as in a were restimulated with (right) or without (left) 1 μg ml−1 of α-CD3 antibody. Flow cytometric contour plots show CPD670 dilution and propidium iodide (PI) staining of Tcon (top) or Treg (bottom) cells gated on (b) nonproliferating cells and (c) proliferating cells. Plots show the frequency of PI-negative viable cells. (d) Statistical representation of % apoptosis of nonproliferating cells (upper panel) and proliferating cells (lower panel). P-value is determined by Mann–Whitney test. Data represent one of two independent experiments showing similar results. (e) Tcons (upper panel) or Tregs (lower panel) were restimulated with 1 μg ml−1 α-CD3 or 0 μg ml−1 α-CD3 under RICD conditions for 30 min. Cells were fixed, stained using α-p65 antibody (red) and 6-diamidino-2-phenylindole (DAPI) (nucleus; blue), and assessed by confocal microscopy. Data from one of three independent experiments are shown.
Tregs and in vitro Tregs resist TCR-mediated RICD and direct Fas-mediated apoptosis
Next, we explored whether TCR-induced RICD was Fas–Fas ligand (FasL) dependent, and therefore induced RICD in the presence or absence of α-FasL antibody. α-FasL antibody abrogated TCR-mediated RICD (Figure 4a), indicating the RICD we observed was Fas–FasL apoptosis. We then analyzed the expression of Fas and FasL at 0 and 6 h after RICD induction. In vitro induced Tregs are referred to as in vitro Tregs in our experiments. Although Fas levels were comparable (Figure 4b), Tregs and in vitro Tregs showed reduced FasL expression compared with Tcons (Figure 4c). Next, we determined whether reduced apoptosis in Tregs was completely attributed to decreased FasL expression, or whether these cells also have intrinsic reduced sensitivity to Fas-mediated apoptosis. We directly crosslinked the Fas molecule using plate-bound α-Fas antibody to induce Fas-mediated apoptosis. Both Tregs and in vitro Tregs showed significantly reduced apoptosis upon direct Fas crosslinking, independent of reduced FasL expression (Figure 4d).
Regulatory T cells (Tregs) and in vitro Tregs show decreased Fas ligand (FasL)/FAS mediated death. (a) % Apoptosis of Tcons (white bars), Tregs (black bars), or in vitro Tregs (gray bars) derived and pooled from spleen/lymph nodes (SPLN/LN) that were stimulated under restimulation-induced cell death (RICD) conditions with or without 1 μg ml−1 of α-CD3 antibody for 12 h, as in Figure 2b. Isotype control antibody or α-FasL antibody (10 μg ml−1) was added 30 min before RICD induction. (b, c) Flow cytometric contour plots of Fas (b) or FasL (c) staining of Tcon (top row), Treg (middle row), or iTreg (bottom row) cells, stimulated as in Figure 2b. Left columns show isotype control staining on Tcon cells. Gates show the frequencies of Fas- or FasL-expressing cells. Data represent one of three independent experiments showing similar results. (d) % Apoptosis of Tcons (white bars), Tregs (black bars), or in vitro Tregs (gray bars) that were restimulated with 1 or 10 μg ml−1 of α-Fas antibody and human interleukin-2 (IL-2) for 8 h. As controls, some cells were restimulated with 0 or 1 μg ml−1 of α-CD3 antibody. P-values are determined by Mann–Whitney test.
RICD resistance is partially dependent on soluble TGF-β1 in Tregs
Next we determined whether a soluble factor might influence RICD resistance in Tcons and Tregs. Therefore, we stimulated Tcons and Tregs, and collected the supernatants from 4-day cultures. We then induced RICD in the presence of Tcon or Treg supernatants. Treg supernatants partially, but significantly, reduced Tcon apoptosis (Figure 5a). We reasoned that IL-10,36 IL-35,37 or TGF-β,38 the most prominent soluble factors produced by Tregs, may underlie apoptosis resistance, and sought to test this hypothesis. However, Il10−/− Tregs were as resistant to RICD as WT Tregs (Figure 5b). Excluding the role of IL-35 in Treg survival, the frequency of Foxp3+ Tregs was normal in Ebi3−/− mice (Supplementary Figure S5). Although previous studies reported the role of TGF-β in Foxp3 expression and the development of Tregs,39 its effect on Treg RICD is undetermined. Based on the antiapoptotic functions of TGF-β in general,40 we examined the role of TGF-β during RICD. We either added TGF-β only during induction of RICD in Tcons or preconditioned them with TGF-β both during the initial stimulation and again during RICD induction. TGF-β reduced RICD moderately but significantly when added only during RICD induction (Figure 5c). However, preconditioning with TGF-β completely abrogated RICD (Figure 5c), and also induced Foxp3 expression (in vitro Tregs) in naive T cells. To confirm the role of TGF-β in conferring resistance to RICD in Tregs, we preblocked TGF-β (1d11 antibody) in Tcons and Tregs during primary stimulation and RICD induction. Blocking TGF-β abrogated RICD resistance partially but significantly in Tregs (Supplementary Figure S6). Similar to Tregs and consistent to a previous publication, in vitro Tregs also showed decreased apoptosis (Figure 5d).24 Although TGF-β did not inhibit proliferation in in vitro Tregs, these cells also showed reduced active caspase levels compared with Tcons upon RICD induction (Supplementary Figure S7A,B). Taken together, these results show that TGF-β plays a crucial role in reducing TCR-induced RICD.
Restimulation-induced cell death (RICD) in Tcons is partially reduced by a soluble factor secreted by regulatory T cells (Tregs) and the reduced apoptosis is dependent on transforming growth factor-β1 (TGF-β). (a) Tcons and Tregs derived and pooled from spleen/lymph nodes (SPLN/LN) were stimulated under RICD conditions for 20 h with indicated concentrations of α-CD3 antibody as in Figure 2b, in the absence (white bars) or the presence of supernatants collected from 4-day Tcon cultures (Tcon supe.) (hatched bars) or those collected from Treg cultures (Treg supe.) (black bars). % Apoptosis, as assessed by annexin-V and propidium iodide (PI) staining. (b) % Apoptosis of wild-type (WT) or Il10 − − Tcons (white bars) or Tregs (black bars) that were stimulated under RICD conditions for 10 h as in Figure 2b. (c) % Apoptosis of WT Tcons restimulated as in Figure 2b in the presence of Treg supe., or increasing concentrations of human TGF-β1 added during RICD induction only. Some Tcons were cultured with 4 ng ml−1 of TGF-β1 during initial stimulation for 4 days (preconditioning (PC)) and during RICD. (d) In vitro Tregs are resistant to RICD. % Apoptosis of Tcons (gray bars) or in vitro Tregs (black bars) that were restimulated with indicated concentrations of α- CD3 antibody for 17 h as in Figure 2b. P-values are determined by Mann–Whitney test.
Absence of TGF-β signaling sensitizes Tregs to TCR-mediated RICD
Based on the antiapoptotic effects of TGF-β1 that we observed above, we hypothesized that Tregs resist RICD because they produce TGF-β1 and are exposed to self-stimulatory TGF-β1 signaling. To determine whether Tregs and in vitro Tregs exhibit increased TGF-β1 signaling, we measured the mothers against decapentaplegic homolog (SMAD) proteins.41 Both Tregs and in vitro Tregs exhibited increased levels of phosphorylated SMAD2 (pSMAD2) (Figure 6a, upper panel) and pSMAD3 (Figure 6a, lower panel), whereas only a fraction of Tcons showed very low levels of these molecules (Figure 6a). To conclusively show that TGF-β1 signaling is required for RICD resistance of Tregs and in vitro Tregs, we utilized the transgenic mice that express a dominant-negative form of the TGF-β receptor II under the direction of the mouse CD4 antigen promoter (Cd4-tgfβr2dntg), in which CD4 cells lack TGF-β signaling.42 We flow cytometrically sorted CD4+CD25−CD44lo naive cells and CD4+CD25+Treg cells from WT or Cd4-tgfβr2dntg to induce RICD. Tregs from WT and Cd4-tgfβr2dntg mice were respectively ∼92 and 80% Foxp3-positive before RICD induction (Supplementary Figure S8A). We found that Cd4-tgfβr2dntg Tcons underwent RICD similar to WT Tcons, and both WT Tregs and WT in vitro Tregs showed reduced apoptosis compared with Tcons (Figure 6b). In striking contrast, Cd4-tgfβr2dntg Tregs and Cd4-tgfβr2dntg naive cells stimulated under iTreg conditions (that did not upregulate Foxp3) showed increased RICD comparable to Tcons (Figure 6b and Supplementary Figure S8B). These data demonstrate that Tregs show increased TGF-β signaling, and that is central to their RICD resistance.
Resistance to restimulation-induced cell death (RICD) is dependent on increased transforming growth factor-β1 (TGF-β1) signaling in regulatory T cells (Tregs) and in vitro Tregs. (a) Tcons, Tregs, and in vitro Tregs derived and pooled from spleen/lymph nodes (SPLN/LN) were stimulated for 4 days with α-CD3, α-CD28, and IL-2, and restimulated in the presence of TGF-β1 for 30 min. Flow cytometric contour plots show Foxp3 and pSmad2 (top) or pSMAD3 (bottom) staining of Tcon (left), Treg (middle), or iTreg (right) cells. (b) % Apoptosis of Tcons, Tregs, or in vitro Tregs from wild-type (WT) or Cd4-tgfβr2dntg mice were restimulated as in Figure 2b without (white bars) or with 1 μg ml−1 (black bars) of α-CD3 antibody for 23 h. Data represent one of three independent experiments showing similar results. P-value is determined by Mann–Whitney test.
Expression profiles of apoptosis-related molecules in Tregs
Next we profiled a panel of apoptosis-related genes using quantitative PCR array analysis of messenger RNA (mRNA) isolated from Tcons and Tregs 3 h after induction of RICD. Il10 was used as a positive control for Tregs. As expected, whereas mRNA level of Il10 was upregulated, Fasl level was downregulated in Tregs (Figure 7a). Some of the Bcl-2 members such as Bcl-2 and Bcl-xl (Bcl2l1) were unchanged or moderately changed compared with Tcons. However, we found that antiapoptotic Cflip (Cflar) mRNA level was upregulated, and proapoptotic Bax and Bok were downregulated in Tregs compared with Tcons. cFLIP is a cytoplasmic protein that is capable of binding to FADD (Fas-associated death domain) and preventing the initiation of the Fas death pathway.43 More importantly, TGF-β has previously been shown to upregulate cFLIP in other cell types.40 Therefore, we validated the expression profiles of cFLIP and other apoptotic genes by assessing their protein levels. Although Tregs and in vitro Tregs upregulated Bcl-2, Bcl-xl, and Bim, their levels were slightly lower, or unchanged, compared with Tcons (Figure 7b). However, supporting our quantitative PCR array data, T and in vitro Tregs showed increased frequency of cFLIPhigh cells, whereas most of the Tcons were cFLIPlow (Figure 7b). These results suggest that TGF-β1-mediated upregulation of cFLIP may also be involved in Tregs and in vitro Tregs in conferring resistance to RICD.
Expression of apoptosis-related genes and proteins in regulatory T cells (Tregs). (a) Tcons and Tregs derived and pooled from spleen/lymph nodes (SPLN/LN) were restimulated as in Figure 2b. Total RNA was prepared 3 h after stimulation to assess the mRNA levels by quantitative PCR (qPCR) array using RT2 Profiler PCR array (indicated by y axes). These data represent one of three independent experiments. (b) Flow cytometric histograms of Tcons (blue), Tregs (red), and in vitro Tregs (green) that were stimulated as above for 12 h, fixed, and stained for intracellular Bcl-xl, Bcl-2, Bim, and cFLIP (cellular FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein).
WT but not Cd4-tgfβr2dntg Tregs undergo reduced apoptosis during oral Candida reinfection in vivo
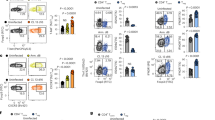
To determine whether increased viability of Tregs during the OPC infection was dependent on TGF-β, we adoptively transferred WT or Cd4-tgfβr2dntg CD4+ cells into Rag1−/− mice and assessed their viability as in Figure 1 in draining lymph nodes. We also examined the CD4 viability in mouse oral intraepithelial and lamina propria leukocytes isolated from oral tissues, the site of infection. The proportion of VD-ef660+ dead cells was significantly lower among WT Foxp3+Tregs than in WT Foxp3−effector cells (Figure 8a,b). However, in mice that received Cd4-tgfβr2dntg CD4+ cells, cell death of the Foxp3+ Tregs was higher than WT Tregs (Figure 8a,b). We then determined the frequency of CD25+Foxp3+ Tregs 2 days after reinfection and found that Cd4-tgfβr2dntg recipient mice showed at least 50% reduced frequency of Foxp3+Tregs than WT recipients (Figure 8c,d). These results demonstrate that Tregs resist apoptosis during later phases of infection and reinfection, and require TGF-β1 signaling for their increased viability.
Increased viability in regulatory T cells (Tregs) during Candida reinfection is transforming growth factor-β1 (TGF-β1) dependent in vivo. (a) Rag1−/− mice (n=11) were reconstituted with CD4+ cells from C57BL/6 or Cd4-tgfβr2dntg congenic mice. Recipient mice in each group were infected and reinfected with phosphate-buffered saline (PBS) sham controls or Candida. Axillary lymph nodes (ALNs), cervical lymph nodes (CLNs), and mouse oral intraepithelial and lamina propria leukocytes (MOIL) were harvested on day 1 after reinfection for examining cell death of Foxp3− Teff in wild-type (WT) cell recipients (left), Foxp3GFP+Tregs in WT cell recipients (middle), and Foxp3GFP+Tregs in Cd4-tgfβr2dntg cell recipients (right) ex vivo by intracellular Foxp3 and VD-ef660 staining (gated on CD4+ cells). (b) Quantification of the frequency of VD-ef660+ dead cells among Foxp3− Teffs (blue circles) and Foxp3+ Tregs (red squares) from ALNs, CLNs, MOIL, and inguinal lymph nodes (ILNs) as in a (gated on CD4+ cells). Flow cytometric dot plots (c) and quantification (d) showing CD25- and Foxp3-expressing cells (Tregs) in WT cell recipients (c, left) and Cd4-tgfβr2dntg cell recipients (c, right) ex vivo (gated on CD4+ cells) 2 days after reinfection as in a. Data represent three independent experiments showing similar results. Statistical significance was determined using Mann–Whitney test.
DISCUSSION
Our data reveal that during late points of OPC infection (Figure 1) and chronic LCMV infection (Supplementary Figure S2), Tregs are exquisitely resistant to apoptosis as compared with conventional CD4+ T cells. During initial phases of acute OPC infection, there was a slight drop in Treg frequencies. This is consistent with a previous observation that demonstrated an infection-induced partial loss of Tregs because of insufficiency in IL-2.44 However, at late time points, whereas most of the effector cells undergo attrition, Tregs survive RICD. We and others have shown that during infections, Toll-like receptor-2 signaling can promote proliferation and accumulation of Tregs in the infected tissues and the draining lymph nodes, and is required to limit immunopathology.31, 45 Although Tregs underwent proliferation, their expansion is less than that of the effector cells, and could not have contributed to increased viability compared with effector cells (Supplementary Figure S1A). It is conceivable that although most effector cells die after infection clearance, Tregs have adopted a survival mechanism to be retained longer in the tissues and limit inflammation and tissue damage. This is consistent to the previous data showing that memory Tregs stay in the system, and serve as more potent suppressors mediating resolution of organ-specific autoimmunity in mice.46
Our data unequivocally show that TGF-β1 is required for RICD resistance in Tregs. Addition of exogenous Treg supernatant or TGF-β1 partially relieved even the Tcon cells from RICD (Figure 5a), demonstrating a partial role of soluble TGF-β1 in decreasing apoptosis during RICD. Moreover, Cd4-tgfβr2dntg Tregs showed complete susceptibility to RICD similar to Tcon levels, substantiating a critical role of TGF-β1 signaling in Treg RICD resistance (Figure 6b). We have shown for the first time that TGF-β1 confers increased viability in Tregs at the site of infection (mouse oral intraepithelial and lamina propria leukocyte tissue) during an oral infection in vivo (Figure 8a), phenotypically and mechanistically recapitulating the in vitro results. It is possible that both soluble TGF-β1 and membrane-bound TGF-β1 (latency-associated peptide) are jointly involved in apoptosis resistance in Tregs. Thus, in the absence of TGF-βRII, unable to respond to both the forms of TGF-β, Tregs showed high susceptibility to RICD. Validating the role of TGF-β further in CD4 cells, TGF-β1-induced in vitro Tregs exhibited RICD resistance as well. Because in vitro Tregs express Foxp3, it can be argued that Foxp3 imparts increased viability in Tregs.27 However, our results showing high RICD susceptibility of FACS-sorted Tregs from Cd4-tgfβr2dntg mice despite Foxp3 expression (Supplementary Figure S8B) rules out this possibility. A potential caveat to the experiment where we adoptively transferred Cd4-tgfβr2dntg Tregs could be the loss of Foxp3 expression in some of the Tregs. Moreover, as we purified these cells using CD4 and CD25 only, there is likely a contamination of CD25+ Teff cells. However, in our viability analyses we gated only on Foxp3+ Tregs (Figure 8a). Therefore, although the starting populations may have been different in terms of Foxp3 expression, even gating exclusively on Foxp3+ Tregs, Cd4-tgfβr2dntg Tregs had poor viability. These results show that despite Foxp3 expression in Cd4-tgfβr2dntg cells, they have poor survival, thus substantiating the role of TGF-β in maintaining Treg survival. Moreover, the overall viable CD4 count of the cells recovered from CLN showed that Cd4-tgfβr2dntg CD4 cell recipient mice had lower CD4 cell numbers than the WT CD4 cell recipients on day 2 after reinfection (Supplementary Figure S9). These results show that Cd4-tgfβr2dntg CD4+ cells did not expand better than WT CD4 T cells at this time point. Therefore, decrease in Foxp3+ cells in Figure 8c,d could not be because of preferential expansion of the activated cell population (Foxp3 negative) on day 2 at which we measured the frequency of Foxp3+ cells.
Thus, we believe that TGF-β1 can confer RICD resistance independently of Foxp3 expression. As shown previously,27 although we observed lower FasL expression in Tregs and in vitro Tregs compared with Tcons (Figures 4b, 7a), we do not attribute the RICD resistance of Tregs solely to reduced FasL expression. This is because human Tregs show little or no reduction in FasL expression compared with Tcons (data not shown),47 yet are resistant to RICD (Figure 2e,f). Moreover, in contrast to the previous findings in naive Tregs,48 activated Tregs and in vitro Tregs showed increased apoptosis resistance even to intrinsic Fas apoptosis that was induced directly using α-FAS antibody (Figure 4d).
We believe that TGF-β1 is involved in cFLIP upregulation in Tregs and in vitro Tregs (Figure 7b), based on a previous study that demonstrated cFLIP induction by TGF-β.40 When we stimulated naive cells using α-CD3 antibody in the presence of IL-2 and TGF-β, the naive T cells not only expressed Foxp3 and became in vitro Tregs, but they also expressed higher levels of cFLIP compared with cells without TGF-β (Figure 7b). These results show that TGF-β is involved in inducing cFLIP along with Foxp3 expression in naive T cells. cFLIP may inhibit intrinsic Fas signaling, contributing to FasL-independent component of RICD resistance in Tregs. Future studies are warranted to investigate cFLIP-dependent survival in Tregs during infections. Thus, our data not only support the previously demonstrated role of TGF-β1 in inducing Tregs and controlling inflammation,39 but also reveal its role in promoting Treg resistance to Fas-dependent RICD in vitro and during infections. Taken together, we show that TGF-β1 contributes to apoptosis resistance mechanism to Tregs and in vitro Tregs during RICD that has implications in controlling immunopathology during microbial infections.
METHODS
Antibodies, reagents, and mice. Purified and fluorochrome-conjugated CD3 (145-2C11), CD28, CD25 (3C7), CD4, Foxp3, and p-Akt antibodies were purchased from eBiosciences (San Diego, CA). Fluorochrome-conjugated annexin-V, Bcl-2, Fas, FasL, Bcl-xl, and Bim antibodies were all purchased from BD Biosciences (San Diego, CA). cFLIP, cleaved caspase-3, caspase-9, and pAkt antibodies were purchased from Cell Signaling Technologies (Danvers, MA). FasL blocking antibody was purchased from R&D Systems (Minneapolis, MN). Fas crosslinking antibody was purchased from Alexis Biochemicals (Farmingdale, NY). Antibody to NF-κB (p65) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse CD4 isolation kits were purchased from Miltenyi Biotec (Auburn, CA) or from Stem Cell Technologies (Vancouver, BC, Canada). Mouse IL-2, IL-10, and human TGF-β were purchased from R&D Systems and BioBasic (Amherst, NY). Complete RPMI-1640 (Hyclone, GE Healthcare, Pittsburgh, PA) supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin (BioBasic), 100 μg ml−1 streptomycin (BioBasic), 1 × Glutamax (Gibco, Thermo Fisher Scientific, Grand Island, NY), and 50 μM β-mercaptoethanol was used for cell cultures. Foxp3GFP reporter mice on Balb/C or C57BL/6 background, and Foxp3YFPcre, Rag1−/−, Il10−/− Ebi3−/−, and Cd4-tgfβr2dntg mice and the appropriate control mice were purchased from Jackson Laboratories (Bar Harbor, ME). Experiments were performed at Case Western Reserve University (CWRU) in compliance with the CWRU School of Medicine Institutional Animal Care and Use Committee, and adhered to national guidelines published in Guide for the Care and Use of Laboratory Animals, 8th Edn, National Academies Press, 2001(protocol no. 2012-0140). Human cells were obtained from peripheral blood mononuclear cells of the healthy donors obtained under an approved protocol, reviewed, and approved by the University Hospitals Case Medical Center institutional review board (protocol no. 03-13-15). All subjects provided written informed consent, and participants <18 years of age were not enrolled in the study.
RICD induction in mouse and human CD4 cells in vitro. CD4+CD25+Foxp3GFP+ Tregs or CD4+CD44low CD25− Foxp3GFP− naive T cells (Tcon) were isolated from Foxp3GFP reporter mice and cultured in flat-bottom 96-well plates in the presence of soluble 1 μg ml−1 α-CD3 and 2 μg ml−1 α-CD28 antibodies and human IL-2 (10 ng ml−1). For induction of Tregs in vitro human TGF-β1 (4 ng ml−1) was added to naive cells during the primary stimulation. After 4–5 days, dead cells were removed using Ficoll-Paque centrifugation, and restimulated with plate-bound α-CD3 antibody and IL-2 (10 ng ml−1) for RICD induction. Where indicated, cells were labeled with cell proliferation dye-670 (CPD670) at the beginning of stimulation. For some experiments, CD4+CD25+ Tregs and CD4+CD44low CD25− Tcons were isolated. For human cell experiments, CD4+CD25hi CD127lo Tregs and CD4+CD44lowCD25− Tcons were used for initial stimulation with 1 μg ml−1 α-CD3 (OKT3) and 2 μg ml−1 α-human CD28 antibodies. After 5 days, RICD was induced using plate-bound OKT3 antibody and IL-2.
Measurement of cell viability by flow cytometry. Cell viability was examined on the basis of forward scatter (FSC) or annexin-V and PI staining of the cells from triplicate culture wells. The percentage of annexin-V+PI+ or FSClo PI+ in flow cytometric plots was used as frequency of dead cells to calculate percentage apoptosis (% Apoptosis). % Apoptosis plotted in all analyses is normalized to the cultures that were stimulated with 0 μg ml−1 α-CD3 antibody, whose cell death was set at 0%. In some experiments, where intracellular Foxp3 expression was also determined, we measured the viability of CD4+ cells using viability dye-efluor660 (VD-ef660, eBiosciences, San Diego, CA). Data were acquired using BD FACS Calibur or BD LSR Fortessa (Franklin Lakes, NJ) analyser cytometers and were analyzed using FlowJo 9.8 software (Tree Star, Ashland, OR).
NF-κB nuclear translocation. After 30-min restimulation, cells were collected for fixation and permeabilization. To determine the nuclear translocation of the NF-κB, cells were cytospun down on the slides, stained using α-p65 antibody, and were assessed by confocal microscopy.
Apoptosis induction in vivo by administration of α-CD3 antibody in mice. Foxp3GFP reporter mice were injected with phosphate-buffered saline or 100 μg of α-CD3 antibody (eBiosciences) by intraperitoneal injection. At 1 day after injection, mice were killed, the spleen and lymph nodes were isolated in single-cell suspensions, and the viability of the cells was assayed using green fluorescent protein (GFP) and PI staining and flow cytometry.
Quantitative reverse transcriptase-PCR array analysis. RT2 Profiler PCR array plates (PAMM-012Z) from Qiagen/SABiosciences (Valencia, CA) were used for profiling apoptosis-related mRNA expression according to the manufacturer’s instructions. Arrays contained 84 apoptosis-related genes and 12 housekeeping genes. Tregs or Tcons were harvested 1 h after RICD stimulation, and RNA was recovered using an EZ-10 RNA isolation kit (BioBasic). Genomic DNA was removed by DNA Away (Ambion; Life Technologies (Grand Island, NY; AM1906)) and complementary DNA was synthesized from total RNA using MuLV reverse transcriptase enzyme (BioBasic) with OligodT primers. Then, 1 μl of complementary DNA was used per array well in one reaction using the SYBR Green quantitative PCR reaction in a real-time PCR machine ABI7900HT (Applied Biosystems, Grand Island, NY).
Oral Candida infection in mice. Mice were infected as previously described.30, 49 Briefly, they were sublingually infected under anesthesia by placing a 3 mm diameter cotton ball saturated with 1 × 108 Candida albicans (CAF2-1) blastospores for 90 min. For experiments involving adoptive transfer of cells, Rag1−/− mice were reconstituted with indicated congenic cells 3–4 days before infection. Mice were reinfected on day 5 after primary infection for assessing the viability during RICD in vivo.
LCMV infection in mice. Foxp3YFP reporter mice were obtained from Jackson Laboratory and were infected intravenously with 2 × 106 PFUs of the LCMV clone 13. Virus was diluted in 200 μl phosphate-buffered saline and injected via retro-orbital route in mice anesthetized with isoflurane. Viral stocks were prepared and viral titers were measured as previously described.50
Statistical analyses. Statistical significance and P-values were determined by Mann–Whitney tests in Prism 6.0 (GraphPad Software, La Jolla, CA). The s.d. values are shown in the data unless otherwise specified.
References
Pandiyan, P., Zheng, L. & Lenardo, M.J. The molecular mechanisms of regulatory T cell immunosuppression. Front. Immunol. 2, 60 (2011).
Sakaguchi, S., Yamaguchi, T., Nomura, T. & Ono, M. Regulatory T cells and immune tolerance. Cell 133, 775–787 (2008).
Cebula, A. et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 497, 258–262 (2013).
Miyara, M., Gorochov, G., Ehrenstein, M., Musset, L., Sakaguchi, S. & Amoura, Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun. Rev. 10, 744–755 (2011).
Whiteside, T.L. Regulatory T cell subsets in human cancer: are they regulating for or against tumor progression? Cancer Immunol. Immunother. 63, 67–72 (2014).
Shi, L.Z. et al. Inhibitory role of the transcription repressor Gfi1 in the generation of thymus-derived regulatory T cells. Proc. Natl. Acad. Sci. USA 110, E3198–E3205 (2013).
Pierson, W. et al. Antiapoptotic Mcl-1 is critical for the survival and niche-filling capacity of Foxp3(+) regulatory T cells. Nat. Immunol. 14, 959–965 (2013).
Lenardo, M.J. Molecular regulation of T lymphocyte homeostasis in the healthy and diseased immune system. Immunol. Res. 27, 387–398 (2003).
Snow, A.L., Pandiyan, P., Zheng, L., Krummey, S.M. & Lenardo, M.J. The power and the promise of restimulation-induced cell death in human immune diseases. Immunol. Rev. 236, 68–82 (2010).
Hildeman, D.A. et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity 16, 759–767 (2002).
Marrack, P. & Kappler, J. Control of T cell viability. Annu. Rev. Immunol. 22, 765–787 (2004).
Russell, J.H., White, C.L., Loh, D.Y. & Meleedy-Rey, P. Receptor-stimulated death pathway is opened by antigen in mature T cells. Proc. Natl. Acad. Sci. USA 88, 2151–2155 (1991).
Schmitz, I., Krueger, A., Baumann, S., Schulze-Bergkamen, H., Krammer, P.H. & Kirchhoff, S. An IL-2-dependent switch between CD95 signaling pathways sensitizes primary human T cells toward CD95-mediated activation-induced cell death. J. Immunol. 171, 2930–2936 (2003).
Hildeman, D.A., Zhu, Y., Mitchell, T.C., Kappler, J. & Marrack, P. Molecular mechanisms of activated T cell death in vivo. Curr. Opin. Immunol. 14, 354–359 (2002).
Bidere, N., Su, H.C. & Lenardo, M.J. Genetic disorders of programmed cell death in the immune system. Annu. Rev. Immunol. 24, 321–352 (2006).
Duke, R.C. & Cohen, J.J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 5, 289–299 (1986).
Strasser, A. The role of BH3-only proteins in the immune system. Nat. Rev. Immunol. 5, 189–200 (2005).
Strasser, A., O'Connor, L. & Dixit, V.M. Apoptosis signaling. Annu. Rev. Biochem. 69, 217–245 (2000).
Pandiyan, P. & Lenardo, M.J. The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol. Direct 3, 6 (2008).
Barron, L. et al. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J. Immunol. 185, 6426–6430 (2010).
Rathmell, J.C. & Thompson, C.B. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell 109, S97–S107 (2002).
Chaudhry, A. & Rudensky, A.Y. Control of inflammation by integration of environmental cues by regulatory T cells. J. Clin. Invest. 123, 939–944 (2013).
Weant, A.E., Michalek, R.D., Crump, K.E., Liu, C., Konopitski, A.P. & Grayson, J.M. Defects in apoptosis increase memory CD8+ T cells following infection of Bim-/-Faslpr/lpr mice. Cell Immunol. 271, 256–266 (2011).
Tischner, D., Wiegers, G.J., Fiegl, H., Drach, M. & Villunger, A. Mutual antagonism of beta and Interleukin-2 in cell survival and lineage commitment of induced regulatory T cells. Cell Death Differ. 19, 1277–1287 (2012).
Ouyang, W., Beckett, O., Ma, Q. & Li, M.O. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity 32, 642–653 (2010).
Banz, A., Pontoux, C. & Papiernik, M. Modulation of Fas-dependent apoptosis: a dynamic process controlling both the persistence and death of CD4 regulatory T cells and effector T cells. J. Immunol. 169, 750–757 (2002).
Weiss, E.M. et al. Foxp3-mediated suppression of CD95L expression confers resistance to activation-induced cell death in regulatory T cells. J. Immunol. 187, 1684–1691 (2011).
Rowe, J.H., Ertelt, J.M. & Way, S.S. Foxp3(+) regulatory T cells, immune stimulation and host defence against infection. Immunology 136, 1–10 (2012).
Zhou, Y. Regulatory T cells and viral infections. Front. Biosci. 13, 1152–1170 (2008).
Pandiyan, P. et al. CD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity 34, 422–434 (2011).
Bhaskaran, N., Weinberg, A. & Pandiyan, P. Th17 inflammation model of oropharyngeal candidiasis in immunodeficient mice. J. Vis. Exp. 96, 52538 (2015).
Filler, S.G., Yeaman, M.R. & Sheppard, D.C Tumor necrosis factor inhibition and invasive fungal infections. Clin. Infect. Dis. 41 (Suppl 3), S208–S212 (2005).
Hughes, P.D., Belz, G.T., Fortner, K.A., Budd, R.C., Strasser, A. & Bouillet, P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity 28, 197–205 (2008).
Yu, X.Z., Zhu, L., Davis, J.E., Tso, J.Y., Hansen, J.A. & Anasetti, C. Induction of apoptosis by anti-CD3 epsilon F(ab')2 in antigen receptor transgenic murine T cells activated by specific peptide. J. Immunol. 157, 3420–3429 (1996).
Lenardo, M.J. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature 353, 858–861 (1991).
Ina, K. et al. Intestinal fibroblast-derived IL-10 increases survival of mucosal T cells by inhibiting growth factor deprivation- and Fas-mediated apoptosis. J. Immunol. 175, 2000–2009 (2005).
Collison, L.W., Pillai, M.R., Chaturvedi, V. & Vignali, D.A. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J. Immunol. 182, 6121–6128 (2009).
Wan, Y.Y. & Flavell, R.A. Regulatory T cells, transforming growth factor-beta, and immune suppression. Proc. Am. Thorac. Soc. 4, 271–276 (2007).
Marie, J.C., Letterio, J.J., Gavin, M. & Rudensky, A.Y. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 201, 1061–1067 (2005).
Schlapbach, R. et al. TGF-beta induces the expression of the FLICE-inhibitory protein and inhibits Fas-mediated apoptosis of microglia. Eur. J. Immunol. 30, 3680–3688 (2000).
Kamato, D. et al. Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell. Signal. 25, 2017–2024 (2013).
Gorelik, L. & Flavell, R.A. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity 12, 171–181 (2000).
Yeh, W.C. et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 12, 633–642 (2000).
Benson, A. et al. Microbial infection-induced expansion of effector T cells overcomes the suppressive effects of regulatory T cells via an IL-2 deprivation mechanism. J. Immunol. 188, 800–810 (2012).
Bhaskaran, N., Cohen, S., Zhang, Y., Weinberg, A. & Pandiyan, P. TLR-2 signaling promotes IL-17A production in CD4+CD25+Foxp3+ regulatory cells during oropharyngeal Candidiasis. Pathogens 4, 90–110 (2015).
Rosenblum, M.D., Gratz, I.K., Paw, J.S., Lee, K., Marshak-Rothstein, A. & Abbas, A.K. Response to self antigen imprints regulatory memory in tissues. Nature 480, 538–542 (2011).
Baatar, D., Olkhanud, P., Sumitomo, K., Taub, D., Gress, R. & Biragyn, A. Human peripheral blood T regulatory cells (Tregs), functionally primed CCR4+ Tregs and unprimed CCR4- Tregs, regulate effector T cells using FasL. J. Immunol. 178, 4891–4900 (2007).
Fritzsching, B. et al. Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood 108, 3371–3378 (2006).
Kamai, Y., Kubota, M., Kamai, Y., Hosokawa, T., Fukuoka, T. & Filler, S.G New model of oropharyngeal candidiasis in mice. Antimicrob. Agents Chemother. 45, 3195–3197 (2001).
Popkin, D.L. et al. Hypomorphic mutation in the site-1 protease Mbtps1 endows resistance to persistent viral infection in a cell-specific manner. Cell Host Microbe 9, 212–222 (2011).
Acknowledgements
We thank Case Comprehensive Cancer center flow cytometry core facility for assisting in FACS cell purification, and access to CFAR flow cytometry core analyzers. P.P. was supported by departmental startup funds from the School of Dental Medicine. This work was also supported by a CTSC core utilization grant to P.P. and SDRC P30AR039750 grant to D.P.
Author contributions
P.P. designed the study, performed experiments, analyzed data, and wrote the manuscript, N.B and C.Q. performed some in vivo experiments, D.P. helped with LCMV infection, A.H. contributed to discussions, and A.W. edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Bhaskaran, N., Quigley, C., Weinberg, A. et al. Transforming growth factor-β1 sustains the survival of Foxp3+ regulatory cells during late phase of oropharyngeal candidiasis infection. Mucosal Immunol 9, 1015–1026 (2016). https://doi.org/10.1038/mi.2015.115
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2015.115
This article is cited by
-
Crosstalk between the oral microbiota, mucosal immunity, and the epithelial barrier regulates oral mucosal disease pathogenesis
Mucosal Immunology (2021)
-
Oral immune dysfunction is associated with the expansion of FOXP3+PD-1+Amphiregulin+ T cells during HIV infection
Nature Communications (2021)
-
FOXP3 protects conventional human T cells from premature restimulation-induced cell death
Cellular & Molecular Immunology (2021)