Abstract
Allergic airway inflammation is driven by the recognition of inhaled allergen by T helper type 2 (Th2) cells in the airway and lung. Allergen-specific cytotoxic T lymphocytes (CTLs) can strongly reduce airway inflammation, however, the mechanism of their inhibitory activity is not fully defined. We used mouse models to show that allergen-specific CTLs reduced early cytokine production by Th2 cells in lung, and their subsequent accumulation and production of interleukin (IL)-4 and IL-13. In addition, treatment with specific CTLs also increased the proportion of caspase+ dendritic cells (DCs) in mediastinal lymph node (MLN), and decreased the numbers of CD103+ and CD11b+ DCs in the lung. This decrease required expression of the cytotoxic mediator perforin in CTLs and of the appropriate MHC-antigen ligand on DCs, suggesting that direct CTL-DC contact was necessary. Lastly, lung imaging experiments revealed that in airway-challenged mice XCR1-GFP+ DCs, corresponding to the CD103+ DC subset, and XCR1-GFP− CD11c+ cells, which include CD11b+ DCs and alveolar macrophages, both clustered in the areas surrounding the small airways and were closely associated with allergen-specific CTLs. Thus, allergen-specific CTLs reduce allergic airway inflammation by depleting CD103+ and CD11b+ DC populations in the lung, and may constitute a mechanism through which allergic immune responses are regulated.
Similar content being viewed by others
Introduction
Allergic airway inflammation is caused by the activation of T helper type 2 (Th2) cells in airway and lung. Upon allergen exposure, Th2 cells produce the hallmark cytokines interleukin (IL)-4, IL-5, and IL-13, which orchestrate the typical allergic airway pathology with mucus production, recruitment of inflammatory cell populations, and consequent tissue damage and airway remodeling.1
Cytokine production by inflammatory Th2 cells is thought to be initiated by recognition of specific allergen in the context of airway antigen (Ag)-presenting cells, with dendritic cells (DCs) playing a predominant role in this process.2 Airway DCs can readily take up allergen in vivo, and upon transfer they can sensitize naïve mice to allergen. Studies using CD11c-diphtheria toxin receptor mice have indicated that DC depletion at the time of airway challenge eliminates the characteristic hallmarks of allergic airway inflammation, including eosinophil infiltration and airway hyper-reactivity,3 and that adoptive transfer of DCs into depleted mice is sufficient to restore inflammation. In addition, localization studies have shown that in sensitized mice intranasal (i.n.) allergen challenge induces a redistribution of DCs to the vicinity of the airways, suggesting an active role of DCs in accessing inhaled Ag during the allergic disease process.4
Allergic airway disease can be modulated by numerous factors, including exposure to airway infection (e.g., BCG, influenza) or environmental stimuli such as endotoxin, which can variously exacerbate, ameliorate or prevent disease.5 These findings are well supported by epidemiological evidence, however, the mechanistic data available are insufficient to explain how these factors interact with the allergic inflammatory process, or how they might be harnessed for the treatment or prevention of disease.
We and others have used rodent models to report that type 1 CD8+ T cells are able to suppress allergic airway inflammation, resulting in decreased eosinophil accumulation, reduced mucus production, and decreased airway hyper-reactivity.6, 7, 8, 9, 10, 11, 12, 13 In particular, allergen-specific CTLs are especially effective at suppressing disease7, 13 and can be primed through appropriate vaccination to reduce airway eosinophilia and mucus production.14 Studies to establish how CTLs might be reducing inflammation showed that expression of the cytotoxic mediator perforin in the CTL population was essential, whereas interferon (IFN)-γ was neither necessary nor sufficient to suppress inflammation.7 These studies point to CTL-mediated killing as being critical for inhibition, however, the precise identity and location of the targets of allergen-specific CTLs were not established.
In this paper, we set out to identify the targets of CTL-mediated killing. We used models of allergic airway inflammation induced through sensitization to ovalbumin (OVA), house dust mite (HDM), or adoptive transfer of Th2 cells15, 16 to examine the effects of allergen-specific CTLs on Th2 cell activation and DCs in airway and lung. We show that allergen-specific CTLs could inhibit the recruitment of Th2 cells to the airway and their production of Th2 cytokines, and at the same time triggered increased apoptosis and loss of local DCs. Lung imaging studies revealed that in the inflamed lung CD11b+ and CD103+ DCs both redistributed to the small airways and co-localized with allergen-specific CTLs. Thus, our data suggest that allergen-specific CTLs can inhibit airway inflammation by inducing DC apoptosis and preventing the activation of disease-mediating Th2 cells.
Results
Allergen-specific CTLs suppress allergic airway inflammation in HDM-primed mice and mice adoptively transferred with OVA-specific Th2 cells
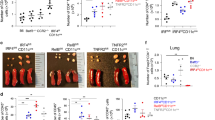
To establish whether allergen-specific CTLs can suppress airway inflammation induced by allergens other than OVA, we used a model of HDM-induced airway eosinophilia (Figure 1a). Mice were sensitized to HDM, and vaccinated with DCs loaded with the MHCI-binding DerP1 peptide epitope FGISNYCQI to induce DerP1-specific CTLs (data not shown). One week after DC immunization, mice were challenged i.n. with HDM. As shown in Figure 1b, mice immunized with DC+DerP1 peptide had lower numbers of airway eosinophils compared with mice that received DCs only, or DCs loaded with the irrelevant OVA peptide SIINFEKL. Total cellularity and the number of T cells in broncho-alveolar lavage (BAL) were also lower in mice immunized with DC+DerP1 peptide, but this was not statistically significant.
Allergen-specific cytotoxic T lymphocytes (CTLs) suppress allergic airway inflammation induced by house dust mite (HDM) or ovalbumin (OVA). (a) C57BL/6 mice were sensitized and challenged with HDM as indicated. HDM-specific CTLs were primed by vaccination with dendritic cells (DCs) loaded with an MHCI-binding peptide from HDM DerP1, or from OVA as a control. (b) HDM-induced airway inflammation was evaluated by quantifying the number of inflammatory cells in broncho-alveolar lavage (BAL) using flow cytometry. Data refer to 6–8 mice/group and are from one of two repeat experiments that gave similar results. (c) C57BL/6 mice were injected with 5 × 106 OVA-specific T helper type 2 (Th2) cells with or without 5 × 106 wild-type (WT) or perforin knock-out (PKO) OVA-specific CTLs, and challenged with OVA by intranasal (i.n.) instillation. Airway inflammation was examined in BAL and lung at the indicated time points. (d) Numbers of airway eosinophils and lung Th2 cells in mice challenged i.n. with 1, 10, or 100 μg OVA were determined by flow cytometry on day 3 as shown in Supplementary Figure S3 online. Data are from one of three experiments (n=3–5) that gave similar results. (e) Histopathological evaluation of lung samples using hematoxylin/eosin (H&E) to reveal cellular infiltration (upper panels), and Alcian Blue and Periodic Acid–Schiff (AB-PAS) to reveal mucus producing goblet cells (lower panels). Bars represent 200 μm (upper panels) and 100 μm (lower panels). Representative sections from one of three experiments, each with 5 mice/group. (f) Interleukin (IL)-4, IL-5, and IL-13 were measured in BAL samples using a Bio-Plex assay. Each symbol corresponds to one mouse. Data are from one of three experiments that gave similar results. Bar graphs show mean number of cells per sample+s.e.m. *P<0.05; **P<0.01; ***P<0.001; as determined using a two-tailed, unpaired t-test (b,d) or analysis of variance with Bonferroni’s correction (f). DerP1p, DerP1 peptide; OVAp, OVA peptide.
Tracking disease-mediating CD4+ T cells in models of allergic airway inflammation induced by in vivo sensitization is difficult due to the low frequency of endogenous Th2 cells in the lung and airway. Therefore, we utilized an adoptive transfer model15, 16 where OVA-specific OTII T cells were activated in Th2 conditions in vitro (Supplementary Figure S1 online) and transferred into naïve recipients before airway allergen challenge (Figure 1c). As shown in Figure 1d (left panel), in mice injected with Th2 cells and no CTLs, the number of eosinophils in BAL remained low after challenge with 1 μg OVA, intermediate after challenge with 10 μg OVA, and was significantly elevated in mice challenged with 100 μg OVA. In contrast, in mice co-injected with Th2 cells and wild-type (WT) CTLs, the number of eosinophils remained low at all the OVA doses used. Mice co-injected with perforin knock-out (PKO) CTLs together with Th2 cells developed high numbers of airway eosinophils, which were comparable to the numbers in mice injected with Th2 cells only, indicating that the previously described role of cytotoxic function in the suppression of airway inflammation7 also extends to this adoptive transfer model. Analysis of the lung cellular infiltrate (Figure 1d, right panel) revealed a similar trend: a dose-dependent increase in the number of Th2 cells with increasing doses of i.n. OVA, which was suppressed by WT CTLs but was unaffected by co-transfer of PKO CTLs. Thus, a dose of 100 μg OVA gave the best response in terms of high airway inflammation, and clear inhibition of inflammation by WT CTLs.
Histopathological analysis of lungs from mice injected with Th2 cells and challenged i.n. with 100 μg OVA revealed a clear inflammatory infiltrate (Figure 1e, upper row) and concomitant goblet cell hyperplasia and mucus production (Figure 1e, lower row). Transfer of WT CTLs together with Th2 cells resulted in reduced inflammation and mucus production compared with mice injected with Th2 cells alone. No response was observed in mice that received no Th2 cells. In addition, the Th2 cytokines IL-4, IL-5, and IL-13 were clearly detectable in BAL from mice injected with Th2 cells only, and CTL co-transfer significantly inhibited the production of these cytokines to levels similar to those observed in naïve mice (Figure 1f).
These data show that adoptive transfer of OVA-specific Th2 cells and CTL reproduces the responses observed in HDM-immunized C57BL/6 mice, and also in OVA-immunized C57BL/6 and PKO mice,7 and provides a platform for investigating the mechanism of those findings.
CTLs prevent the increase in total and cytokine producing Th2 cells in the lung of allergen-challenged mice
To assess cytokine production by allergen-specific CD4+ T cells in the lung, we used adoptive transfer of Th2 cells generated from 4C13R-OTII mice, in which the fluorescent markers AmCyan and dsRed report expression of IL-4 and IL-13,17 respectively. In mice injected with 4C13R-Th2 cells, BAL cytokine levels resembled those observed in Th2-induced inflammation, and were similarly decreased by CTL treatment (Figures 2a vs. 1f).
The number of total and cytokine-producing T helper type 2 (Th2) cells in the lung is significantly reduced by cytotoxic T lymphocytes (CTLs). C57BL/6 mice were injected with Th2 cells and CTLs as in Figure 1c, except that 4C13R-OTII cells were used instead of OTII. Mice were challenged with ovalbumin (OVA) by intranasal (i.n.) instillation 1 day later. Control mice (Th2 PBS) were injected with Th2 cells and challenged i.n. with PBS. (a) Interleukin (IL)-4, IL-5, and IL-13 levels were measured in broncho-alveolar lavage (BAL) 1 day after i.n. OVA challenge. Bar graphs show mean+s.e.m., each symbol corresponds to one mouse. (b) Representative dot plot showing fluorescent reporting of IL-4 and IL-13 in lung samples 1 day after i.n. OVA challenge. Only Vα2+ CD4+ events are shown. (c) Numbers of total transferred OTII T cells, and IL-4 and IL-13 fluorescent reporter+ OTII T cells, in lungs as determined by flow cytometry 1 day after i.n. OVA challenge. Gating is shown in Supplementary Figure S3 online. Bar graphs show mean numbers+s.e.m.; each symbol refers to one mouse. Data are from one of two experiments that gave similar results. (d) and (e): as in (b) and (c), respectively, except that data refer to day 3 after i.n. OVA challenge. *P<0.05, **P<0.01. As determined using a two-tailed, unpaired t-test.
We examined lung 4C13R-Th2 cells for fluorescent reporting of IL-4 and IL-13 1 day after i.n. allergen exposure, when peak cytokine production is observed, and on day 3 which corresponds to the peak of cellular infiltration in the lung. Mice injected with 4C13R-Th2 cells and challenged i.n. with phosphate-buffered saline (PBS) were used to establish the background of the response. On day 1, the number of infiltrating 4C13R-Th2 cells in the lungs of all groups was similarly low regardless of i.n. allergen exposure (Figure 2b). Nonetheless, a few 4C13R-Th2 cells were already reporting cytokine production, with the number of cells reporting IL-4 only, or IL-4 and IL-13, lower in the CTL-treated group compared with the Th2-only control (Figure 2c). On day 3, 4C13R-Th2 cells in the Th2-only group were increased in number compared with day 1 (Figure 2d) and many of those cells were expressing fluorescent reporters (Figure 2e). The increase was much smaller in mice treated with CTLs. Thus, CTL treatment ameliorates airway inflammation by reducing the accumulation of Th2 cells in the lung, and their cytokine production.
CTL treatment leads to increased expression of pro-apoptotic caspases in DCs
To identify the potential targets of CTL-mediated killing in mice undergoing airway challenge, we used the fluorogenic substrate FLIVO, which forms covalent bonds with active caspases in pre-apoptotic cells thus enabling for their direct identification by flow cytometry. In these experiments, we used the sensitization model of allergic airway inflammation (Figure 3a) to ensure visualization of any cell populations potentially induced through immunization. Live FLIVO+ cells were easily identified in mediastinal lymph node (MLN); in contrast, the presence of auto-fluorescent cells made the identification of pre-apoptotic cells in lung difficult, and this tissue was not further examined.
Increased activation of pro-apoptotic caspases in dendritic cells (DCs) after cytotoxic T lymphocyte (CTL) treatment. (a) Mice were sensitized and challenged with ovalbumin (OVA) according to the indicated schedule. OVA-specific CTLs were given 1 day before OVA challenge. One day after OVA challenge, mice were treated with FLIVO to identify apoptotic cells, and were killed 1 h later. (b) Proportion of FLIVO+ mediastinal lymph node (MLN) cells. The bar graph shows the mean percentage of apoptotic cells/LN for 3–5 mice/group+s.e.m. (c) Identification of pre-apoptotic cells by flow cytometric analysis of MLN from FLIVO-treated (top panel) and untreated (lower panel) mice. 4′-6-Diamidino-2-phenylindole (DAPI)-high cells were excluded from analysis. (d) Characterization of the pre-apoptotic population by flow cytometry. FLIVO+ cells were gated as in c and examined for expression of CD11c and MHCII (top panel). Expression of CD11c and MHCII in the total LN population is shown as a comparison (lower panel). (e) Proportion of MHCIIhi migratory DCs in the FLIVO+CD11c+MHCII+ population (top panel) compared with the total MHCIIhi cells in the LN (lower panel). Data are from one of two experiments, each with 3–5 mice per group, that gave similar results. ***P<0.001 as determined using one-way analysis of variance with Bonferroni’s correction. PKO, perforin knock-out.
A comparison of the proportion of lymph node (LN) cells displaying active caspases revealed that these were increased in the CTL-treated group compared with the OVA/Alum and the PKO CTL-treated groups (Figure 3b). In CTL-treated mice, the majority of FLIVO+ cells displayed the FSC/SSC and CD11c+MHCII+ characteristics typical of DCs (Figure 3c–e). CD11c+MHCII+ cells represented about 66.1% of the FLIVO+ population, but only about 3.4% of the total LN cells (Figure 3d, top vs. lower panel). In addition, over 60% of the FLIVO+ DCs were MHCIIhi, compared with about 45% MHCIIhi cells in the total DC population (Figure 3e). These results suggest that CTL-mediated killing preferentially affects the migratory DC population in MLN.
CTLs induce a perforin-dependent decrease in the number of lung DCs
To establish whether lung DCs were also affected by CTL treatment, we examined the numbers of DCs 1 day after i.n. challenge. Three populations were examined: the CD11b+ and CD103+ DCs, found in the steady state, and the monocyte-derived CD11b+CD64+ DCs, found during inflammation (Figure 4a). Each of these DC subsets takes up allergen after i.n. challenge (ref. 18 and data not shown). The number of DCs in each subset was higher in inflamed lungs compared with non-inflamed (Th2 only vs. no cells, Figure 4b). This increase was largely reversed by transfer of WT CTLs, but not by PKO CTLs (Figure 4b). These data suggest that CTL treatment induces a perforin-dependent decrease in lung DC numbers.
Cytotoxic T lymphocyte (CTL) treatment induces a perforin-dependent decrease in lung dendritic cells (DCs). C57BL/6 mice were injected with activated T helper type 2 (Th2) cells and CTLs as in Figure 1c, and challenged with ovalbumin (OVA) by intranasal (i.n.) instillation 1 day later. Lung DCs were examined 1 day after i.n. OVA challenge. (a) Representative gating of total lung DCs by CD11c and MHCII staining, and identification of the CD103+, CD11b+CD64−, and CD11b+CD64+ DC subsets by flow cytometry. The full gating strategy is shown in Supplementary Figure S4 online. FMO, fluorescence minus one. (b) Numbers of CD103+, CD11b+CD64−, and CD11b+CD64+ DCs per lung were determined as shown in a. Bar graphs show mean DC numbers+s.e.m. in the indicated experimental groups. Data are from one of two experiments, each with 3–5 mice per group, that gave similar results. **P<0.01, ***P<0.001, ****P<0.0001; NS, not significant; as determined using one-way analysis of variance with Bonferroni’s correction. PKO, perforin knock-out.
To address whether the decreased DC numbers in CTL-treated mice was simply related to the degree of lung inflammation, we devised an experimental model where DC populations could be compared within the same mouse, and between groups of mice in which the degree of airway inflammation was expected to be similar. We generated mixed bone marrow (BM) chimeras using mixtures of WT H2-Kb (CD45.1+) and H2-Kbm1 (bm1, CD45.2+) BM cells. DCs expressing the H2-Kbm1 molecule are unable to interact with OTI CTLs, but express WT MHCII thus retaining the ability to present Ag to CD4+ T cells, and promote allergic inflammation (Figure 5a and data not shown). Chimeric mice were injected with activated OTI CTLs and Th2 cells as described in Figure 1c, and 1 or 2 days after allergen challenge, mice were killed to determine the ratio of WT (CD45.1+) to bm1 (CD45.2+) DCs in the lungs and MLN. This ratio was normalized to the CD45.1+/CD45.2+ ratio of CD11c+ cells in the spleen of each mouse, to account for potential variations in the take of each donor BM population in individual mice.
Treatment with cytotoxic T lymphocytes (CTLs) targets CD11c+CD11b+CD64− lung dendritic cells (DCs). Mixed BM chimeras were generated by co-injecting wild-type (WT) B6-SJ (CD45.1+) and bm1 (CD45.2+) BM into B6-SJ lethally irradiated hosts. Chimeric mice were injected with T helper type 2 (Th2) cells and OTI CTLs and challenged with ovalbumin (OVA) intranasal (i.n.) as described in Figure 1c. DC populations in the lung and mediastinal lymph node (MLN) were examined by flow cytometry 1 or 2 days later using the gating shown in Supplementary Figure S4 online. (a) Experimental concept. Airway inflammation is maintained in chimeric mice as bm1 Ag-presenting cells can present OVA to OTII cells but are not recognized by OVA-specific CTLs. (b) Normalized ratio of WT (CD45.1+) to bm1 (CD45.2+) DCs in the lung of chimeric mice. DC subsets were identified by flow cytometry as in Figure 4a; ratios were normalized to the ratio of CD45.1/CD45.2 DC in the spleen to account for any differences in the take of BM cells in individual mice. Bar graphs show mean ratio+s.e.m.; each symbol represents one mouse. (c) As in b, except that normalized ratios of DC in MLN are shown. Combined data from two experiments, each with 4 or 5 mice per group, are shown. *P<0.05, **P<0.01, ****P<0.0001 as determined using a two-tailed, unpaired t test.
CTL treatment resulted in decreased ratios of WT to bm1 DCs for both the CD103+ and CD11b+CD64− DC populations in the lung, but reached statistical significance only in the case of the CD11b+CD64− DC population (Figure 5b). The ratios for both the CD103+ and CD11b+CD64− DC populations were also decreased in the MLN (Figure 5c). Therefore, within the same host, DCs expressing the appropriate ligand were preferentially targeted by CTL treatment.
These results suggest that CTLs suppress allergic inflammation by depleting airway DCs through two separate mechanisms: directly by targeting DCs expressing the appropriate cognate ligand and indirectly by reducing the inflammation-dependent increase in the number of DCs in the lung and airway. These results are consistent with the key role of DCs during airway inflammation induced by different allergens including OVA,3 HDM (Supplementary Figure S2A), and in the OVA adoptive transfer model used here (Supplementary Figure S2B).
XCR1-GFP+ and CD11c+XCR1− DCs redistribute to the vicinity of the small airways in the inflamed lung and co-localize with allergen-specific CTLs
Previous studies have reported that CD4+ T cells localize together with allergen-loaded CD11b+ DCs in the inflamed lung,4 however, no information is available on CD8+ T cells, or the localization of CD11b+ vs. CD103+ DCs during inflammation. We used transfer of tandem dimer Tomato (tdTomato)+ OTI CTLs into XCR1GFP recipient mice, in which expression of the XCR1-GFP marker identifies CD103+ DCs19 and differentiates them from the total lung DC population. Using fresh tissue slices from lungs of OVA/Alum primed mice, we observed that, by 18 h after airway challenge, XCR1-GFP+ DCs had redistributed to form large clusters, and that many tdTomato CTLs were also localizing to the same areas (Figure 6a). A similar clustering has been reported by other authors in the airway-adjacent regions of the inflamed lung, and was shown to also include CD4+ T cells.4
XCR1+ and CD11c+XCR1− lung dendritic cells (DCs) co-cluster with cytotoxic T lymphocytes (CTLs) in ovalbumin (OVA) primed and challenged mice. XCR1GFP mice were primed intraperitoneally with OVA/Alum or Alum only, OVA challenged on day 7, and killed 18 h after intranasal (i.n.) challenge. OTI CTL-expressing tdTomato were injected 1 day before i.n. challenge. Tissues were examined by fluorescent microscopy; cell populations in all images are identified by the colors shown in c. (a) Fresh lung tissue from XCR1GFP mice was sliced and examined for localization of GFP+ DCs and tdTomato OTI CTLs. Bars correspond to 200 μm. (b) Tissue sections from OVA-challenged mice showing that XCR1-GFP+ DCs localize around the epithelia of large airways, whereas CD11c+XCR1− DCs are found diffuse in the lung parenchyma. Bars correspond to 200 μm. (c) High-magnification images of tissue sections from OVA-challenged mice showing the clustering of XCR1-GFP+ DCs, CD11c+XCR1− DCs, and CTLs to the inflamed areas around the small airways. Bars correspond to 100 μm.
Analysis of frozen lung sections revealed that there was little or no DC clustering around the larger airways: XCR1-GFP+ DCs were preferentially located in the airway wall, as reported,20 whereas CD11c+XCR1− DCs and CTLs had a more diffuse distribution (Figure 6b). In contrast to the large airways, high-magnification images of the small airways showed clustering of XCR1-GFP+ and CD11c+XCR1− DCs with each other and with tdTomato+ CTLs (Figure 6c).
To further document whether CTLs were co-localizing with XCR1-GFP+ DCs, CD11c+XCR1− DCs, or both, we examined lung sections from the same mice using higher magnification. For these studies, we selected lung areas where DC clustering was less prominent, as they enabled a more accurate evaluation of cell–cell contacts. Depending on the field under examination, tdTomato+ CTLs could be found immediately adjacent to XCR1-GFP+ DCs (Figure 7a) or CD11c+XCR1− DCs (Figure 7b), suggesting that either DC population was interacting with CTLs.
Cytotoxic T lymphocytes (CTLs) are found in the vicinity of both XCR1-GFP+ and CD11c+XCR1− dendritic cells (DCs) in ovalbumin (OVA)-challenged mice. (a,b) XCR1GFP mice were immunized and challenged as in Figure 6, and killed 18 h later for microscopic analysis of DCs and T-cell subsets. (a) Tissue section showing tdTomato CTLs mainly co-localizing with XCR1-GFP+ DCs. Bars correspond to 100 μm. (b) Tissue section showing tdTomato CTLs mainly co-localizing with CD11c+XCR1− DCs. Bars correspond to 100 μm.
Discussion
In this paper, we show that allergen-specific CTLs suppress airway inflammation by inducing DC apoptosis, through a mechanism that is perforin dependent and requires recognition of the appropriate MHC ligands on DCs. DC apoptosis was most likely initiated in the lung, as apoptotic DCs in MLN expressed a migratory DC phenotype. In addition, DCs in CTL-treated lungs were reduced in numbers, and were found to localize to the same spatial compartment as the CTLs thus providing the opportunity for direct contact between these cells. CTL treatment also reduced the number of CD4 cells in the airway and their production of Th2 cytokines, a result that is similar to those obtained in CD11c- diphtheria toxin receptor mice depleted of DCs at the time of airway challenge3 (Supplementary Figure S2). Thus, CTLs suppress inflammation by clearing allergen-loaded DCs in the lung, and reducing the local activation of Th2 cells and the resulting airway disease.
The effects of CTL treatment were already clearly detectable at 24 h after i.n. allergen challenge, with reduced numbers of cytokine-producing Th2 cells in the lung parenchyma, and decreased cytokine levels in BAL. This implies that the targeting of DCs by CTLs was already occurring within the first 24 h of the response. Such early targeting would effectively interrupt the Th2-dependent cascade of events that leads to airway inflammation, and translate relatively small changes in DC numbers into larger effects on Th2 cytokine levels and lung pathology.
A number of studies in experimental models have shown that CTLs can reduce lung inflammation and airway hyper-reactivity.6, 7, 8, 9, 10, 11, 12, 13, 14 We focused our study on allergen-specific CTLs as they are profoundly inhibitory, and they could potentially form the basis of allergen-specific immunotherapies. Our previous work showed that Ag-specific CTLs can inhibit the priming of naïve CD4+ T cells in vivo,21 and that their cytotoxic function is critical to their ability to inhibit allergic airway inflammation.7 Similarly, IFN-γ KO CTLs show impaired killing and are unable to inhibit inflammation (ref. 22 and our unpublished observation). Therefore, one of the goals of this study was to identify the potential targets of CTL-mediated cytotoxicity. An unbiased search for pre-apoptotic cells in CTL-treated mice revealed that the majority expressed a phenotype consistent with migratory DCs. Further experiments using normal mice and mixed BM chimeras confirmed that DC numbers were decreased in the lung. Together with previous work showing that DCs are susceptible to CTL-mediated killing in vitro,23, 24 our data consistently point to allergen-loaded DCs as the targets of CTL activity.
Our flow cytometry data indicate that CTL treatment decreased DC numbers in both lung and MLN. This result makes it difficult to establish whether the functionally important DC targeting occurred in the lung or in the MLN. The observation that, immediately after i.n. sensitization, CD4+ T cells failed to accumulate and secrete cytokines in lung suggests that an important part of the CTL function must have been in the lung itself. Decreased DC numbers in lung would then be reflected in the MLN through DC migration. Experiments using the fluorescent tracer FLIVO were consistent with this possibility, and indicated that pre-apoptotic cells in MLN mostly expressed a CD11cint MHChi phenotype characteristic of migratory DCs. For these DCs, the apoptotic process may well have started in the lung, as FLIVO inhibits the apoptosis-inducing pathways to facilitate identification of otherwise short-lived apoptotic cells. Our previous data with BM-DCs injected s.c. also suggest that DCs are preferentially targeted at the site of injection, and not in the LN.25 Thus, we propose that CTLs can target DCs in the lung, and that decreased DC numbers in MLN are simply a consequence of earlier lung events.
The possibility that in vivo DCs may become targets of specific CTLs has been documented in previous studies using viral models.26, 27 During infection, DCs might simply become virus-infected and be subsequently cleared by CTLs. Our study is the first to report an effect of CTL killing during immune responses to a protein Ag such as OVA, which cannot be presented by DCs via the endogenous pathway but requires cross-presentation. Cross-presentation of exogenous Ag is thought to be the selective property of specialized subsets of DCs, the lymphoid-resident CD8+ DCs, and the migratory CD103+XCR1+ DCs.28 Somewhat unexpectedly, we found that the CD103+ DCs were not the only subset affected by CTL activity, and that CD11b+ DC numbers were also decreased. As CD11b+ DCs are thought to have a key role in presenting allergens to Th2 cells in the lung,18 the depletion of these DCs appears consistent with the inhibitory effect of CTLs on airway inflammation. We did not attempt to distinguish whether in our model CD11b+ DCs were indeed cross-presenting inhaled Ag, a property they can acquire when activated by appropriate stimuli,29 or whether they might acquire pre-processed Ag from other cells, including the CD103+ DCs, via mechanisms such as cross-dressing. In this respect, we are the first to report that, in airway challenged mice, both the CD11b+ and the CD103+XCR1+ DC subsets were redistributing to the proximity of the small airways, where they were found close to each other, to effector CTLs, and presumably to CD4+ T cells.4 This localization might favor the exchange of Ag and/or Ag-MHCI complexes between DC subsets, and has already been proposed to favor the exchange of Ag from the alveolar space to parenchymal DCs.4 The mechanism of such Ag exchange is still unclear, and the chemokines or other molecular cues that control DC redistribution in the lung remain to be characterized. In the skin, DC clustering with perivascular macrophages has recently been reported to be important for local activation of effector T cells,30 suggesting that a similar mechanism may be conserved across tissues and could be critical for local effector immune responses.
Several experiments in this paper involved adoptive transfer of OVA allergen-specific CD4+ and CD8+ T cells appropriately activated in vitro. Use of this model was necessary to enable a precise quantitation of allergen-specific, IL-4- and IL-13-producing CD4+ T cells in the lung. Importantly, the conditions for adoptive transfer experiments were chosen to closely recapitulate findings in normal C57BL/6 or PKO mice primed with allergen to elicit a Th2 response, and vaccinated with DC+allergen peptides7(Figure 1) or α-Gal-Cer-peptide conjugates14 to elicit a CTL response. In all cases, allergen-specific CTLs were found to mediate suppression of airway inflammation, whereas CTLs of irrelevant specificity had a modest or undetectable effect.7, 14 These similarities suggest that allergen-specific CTLs have similar effects across different allergic airway models, a possibility that is also supported by the similar outcomes of DC depletion during allergic responses to different inhaled allergens.
The severity of allergic airway disease in humans is affected by several factors, with CD8+ T cells mediating potentially opposing functions in this process.31 Although “type 2” CD8+ T cells exacerbate disease, allergen-specific and non-specific “type 1” CD8+ T cells ameliorate inflammation in mouse studies.6, 7, 8, 9, 10, 11, 13 CTLs that are not allergen specific can suppress inflammation via cytokine secretion induced by microbial signals.8, 9 In contrast, allergen-specific CTLs show a more powerful inhibitory activity and do not require microbial signals (Enomoto et al, in preparation) or IFN-γ,7 but suppress inflammation by cytotoxic targeting of DC populations in the airway. Although cytokines such as IL-12 and IFN-γ are not essential for the inhibitory function of allergen-specific CTLs, they are critically important during the early phases of CD8 T-cell activation when they promote acquisition of cytotoxic activity and effector function.13, 22 An inflammatory environment, such as experienced by children raised in a farm environment,32 may similarly support the priming of protective CTL responses to inhaled allergens during early life,33 as suggested by experiments where inhaled OVA was found to elicit efficient CD8+ T-cell priming if given at the time of viral infection.34 Interestingly, in patients with severe asthma, circulating CD8 T cells express an activated profile with reduced levels of cytotoxic mediators,35 which would be consistent with the inability of any allergen-specific cells to suppress inflammation. Preserving the “type 1” polarization of allergen-specific CTLs may enable durable protection from asthmatic inflammation.
In conclusion, we present evidence that immunotherapies based on MHCI-binding allergen epitopes may prove beneficial in the treatment of allergic airway disease, by reducing allergen-loaded DCs in the airway and the lung, and decreasing local Th2 cell activation and subsequent inflammation. Vaccines eliciting such a response are being developed for treatment of allergies14 and could be tested in clinical studies. Our findings also offer insights on a cross-talk between CTLs, DCs and CD4+ T cells that has implications for the regulation of immune responses in vivo,36 and may have potential applications in the treatment of other inflammatory diseases.
Methods
Mice. C57BL/6J and B6.SJL-Ptprca Pepcb/BoyJ (B6-SJ) mice were originally from the Jackson Laboratory (Bar Harbor, ME). The T cell receptor transgenic strains OTI (H-2Kb+OVA257–264), PKO-OTI (backcrossed to C57BL/6-Prf1tm1Sdz/J), tdTomato OTI (crossed to B6;129S6-Gt(ROSA)26Sortm14(CAG−tdTomato)Hze/J, and CAG-Cre37), OTII (IAb+OVA323-339) and 4C13R-OTII (crossed to 4C13R reporter mice)17 were used as T-cell donors. All mice were bred and used at the Malaghan Institute of Medical Research or RIKEN Center for Integrative Medical Sciences. Experimental protocols were approved by the respective animal research committees and performed according to the institutional guidelines.
Generation of XCR1GFP mice. A targeting vector was designed to replace the mouse Xcr1-coding sequences with a gene encoding green fluorescent protein (GFP) followed by a polyadenylation signal derived from bGHpA. A neomycin-resistance gene (neo) driven by the MC1 promoter and flanked by the yeast FLP recombination target sequences was used as a selection marker, with Herpes Simplex virus thymidine kinase gene (HSV-TK) for negative selection. The similar Xcr1-targeting vectors have been reported.38 C57BL/6J ES cells were electroporated with the linearized targeting vector; correctly targeted clones were aggregated with BALB/c morulas. Chimeric male offspring were mated with C57BL/6J female mice.
Generation of BM chimeras. Recipient mice were irradiated with two doses of 5.5 Gy given 5 h apart, and injected with 10 × 106 BM cells on the following day. For mixed BM chimera experiments, recipient mice were injected with anti-CD4 (clone GK1.5), anti-CD8 (clone 2.43), and anti-NK1.1 (clone PK136, all generated in-house) on day −1 and +1 with respect to BM transfer. Mice were treated with 2 mg ml−1 neomycin sulfate in drinking water throughout the experiment. H2-Kbm1 (bm1) BM cells were kindly provided by A/Prof Alex McLellan, Otago University, NZ.
Culture media and reagents. All cultures were in complete IMDM (IMDM, 2 mM glutamax, 1% penicillin–streptomycin, 5 × 10−5 M 2-ME, and 5% FBS, all from Invitrogen, Auckland, NZ). OVA257–264, OVA323-339, and DerP1111-119 peptides were from Mimotopes, Clayton, Victoria, Australia. Lipopolysaccharide from Escherichia coli was from Sigma, St Louis, MO. Low endotoxin EndoGrade OVA was from Hyglos GmbH, Bernried, Germany. HDM was a freeze-dried extract from Dermatophagoides pteronyssinus (Greer, Lenoir, NC).
Culture and adoptive transfer of CD4+ Th2 cells and CTLs. BM-DCs were generated in cultures with granulocyte macrophage colony-stimulating factor and IL-4 as described,7 and activated by adding 100 ng ml−1 lipopolysaccharide during the last 24 h of culture. To generate Th2 cells, LN cells from OTII donors were co-cultured with OVA323-339-loaded BM-DCs in medium supplemented with 20 ng ml−1 human rIL-2 and 62 ng ml−1 mouse rIL-4 for 5 days. To generate CTLs, LN cells from OTI donors were co-cultured with OVA257–264-loaded BM-DCs for 4 days, then expanded for a further 2 days in 100 U ml−1 human rIL-2 as described.7 Cells were harvested at the end of culture and 5 × 106 cells were injected i.v. into recipient mice. Mice were challenged i.n. with low endotoxin EndoGrade OVA (Hyglos GmbH, Bernried, Germany) 1 day later.
For lung imaging experiments, 50 × 106 tdTomato -OTI or non-fluorescent OTII cells were resuspended in 10 ml RPMI-1640 media (Wako Pure Chemical Industries, Osaka, Japan) supplemented with 10% FCS, 50 μM 2-ME, 1:100 P/S, and cultured with 2 × 106 anti-CD3/CD28 Dynabeads/ml (Gibco, Life Technologies, Tokyo, Japan). Both cultures were supplemented with 20 ng ml−1 IL-2. OTII cultures were also supplemented with 62 ng ml−1 IL-4. Cultures were split and replenished with cytokines on day 3, and harvested on day 5.
Sensitization and challenge. Mice were sensitized by intraperitoneal (i.p.) injection of 2 μg OVA (Grade V, Sigma-Aldrich, St Louis, MO) in 1.36 mg of Alu-Gel-S (SERVA GmbH, Heidelberg, Germany) on days 0 and 14, and challenged i.n. with 100 μg EndoGrade OVA on day 24.7 For HDM experiments, mice were primed i.p. with 40 μg HDM in Alu-Gel-S on day 0, and challenged i.n. with 100 μg HDM on day 17. Intraperitoneal priming was used as this results in a stronger inflammatory response in BAL upon i.n. challenge. To generate DerP1-specific CTLs, mice were injected i.v. with 2 × 105 BM-DCs loaded with DerP1111-119 peptide.39
Experiments on XCR1GFP mice used a short protocol where mice were sensitized i.p. with 100 μg OVA in alum on day 0, and challenged i.n. with OVA on day 7.40 Mice were killed 18 h later for microscopic analysis.
To detect apoptotic cells, the fluorogenic substrate FLIVO (ImmunoChemistry Technologies, Bloomington, MN) was injected i.v. 1 day after i.n. challenge, and 1 h before mice were killed.
Analysis of lung inflammation. BAL was carried out with 1 ml PBS on lethally anesthesized mice as described.7 Recovered cells were counted and stained for flow cytometry; BAL cytokines were measured using a BioPlex Pro Mouse Cytokine Group I, 7-plex assay (Bio-Rad Inc., Hercules, CA). Lung cell suspensions for analysis of cellular infiltrates and lung histopathology were prepared as described.7
Flow cytometry. Cell preparations were resuspended in PBS containing 10 mM EDTA, 0.01% NaN2 and 2% FBS, and pre-incubated in anti-FcγRII (2.4G2, affinity purified from hybridoma culture supernatant) to block Fc receptors. The following antibodies were used for BAL analysis: anti-SiglecF-PE (E50-2440, BD, San Jose, CA), anti-MHCII (3JP-AF488, prepared in-house), anti-CD11c-PerCP-Cy5.5 (N418, BioLegend, San Diego, CA), anti-CD3 PE-Cy7 (17A2, BioLegend). For lung and LN cell suspension analyses, anti-CD11c-PE-Cy7 (HL3, BD), anti-CD103 Biotin+SA PE-Texas Red (M290 BD,+BD), anti-CD11b PerCP Cy5.5 (M1/70, BD), and anti-CD64 APC (X54-5/7.1, BioLegend) were used to identify DC subsets. MHC-II was detected using either AF488 (3JP, prepared in house), APC Cy7 (M5/114.15.2, BioLegend), Pacific Blue (M5/114.15.2, BioLegend), or Alexa Fluor 647 (3JP, generated in-house), depending on the requirements of the specific panel as shown in Supplementary Figures S3 and S4. CD45.1 APC eFluor780 (A20, eBioscience, San Diego, CA) and CD45.2 PE (104, eBioscience) were used to differentiate cells of WT or bm-1 origin in the lung and MLN. Identification of T cells in the lung was carried out using CD3 PE-Cy7 (17A2, BioLegend), CD8 PerCP Cy5.5 (53-6.7, BD), CD4 FITC (RM4-5, eBioscience), or CD4 Pacific Blue (RM 4-5, BD), Vα2 APC (B20.1, BioLegend) and CD45.1 APC (A20, eBioscience). DAPI (Invitrogen, Auckland, NZ) was used to exclude dead cells. Analysis of in vitro generated CD4+ and CD8+ T cells involved staining with CD4 Pacific Blue (RM 4-5, BD), CD8 AF700 (53-6.7, eBioscience), CD44 APC eFluor780 (IM7, eBioscience), CD69 AlexaFluor 488 (H1.2F3, BioLegend), CD62L PE-Cy7 (MEL-14, BioLegend), Vα2 APC (B20.1, BioLegend) or Vα2 FITC (B20.1, eBioscience), and Vβ5.1,5.2 PE (MR9-4, BD). Specific combinations of fluorophores used and full gating ancestry for each flow experiment are depicted in Supplementary Figures S1, S3 and S4. Samples were run on a LSRII multicolor flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star Inc, Ashland, OR).
Fluorescence microscopy. Lung tissues were fixed in 4% paraformaldehyde for 2 h, washed in PBS and snap-frozen in Tissue-Tek OCT compound (Sakura, Tokyo, Japan). 12 μm sections were affixed to MAS-GP–coated slides (Matsunami Glass, Osaka, Japan) and stained at room temperature for 2 h using anti-CD11c-APC (N418, eBioscience) and rabbit anti-GFP (MBL, Life Technologies, Tokyo, Japan) followed by goat anti-rabbit IgG-AF488 (Life Technologies). Nuclei were stained with 3 μM DAPI (Sigma-Aldrich). Images were acquired on BZ-9000 (Keyence, Osaka, Japan), and processed with Pixelmator (2.1.2, Pixelmator Team Ltd, Vilnius, Lithuania). For visualization of XCR1-GFP+ DCs and tdTomato+ OT-I cells, fresh lung tissues were sliced with razor blades, stained with anti-CD45.1-Cy5 (clone A20, eBioscience), and imaged with the TCS SP5 confocal laser microscope (Leica Microsystems, Wetzlar, Germany).
Statistical analysis. Data are expressed as mean±s.e.m. Data were tested for normality using the D’Agostino-Pearson omnibus test. Statistical analyses were performed using Prism 5 (GraphPad Software, San Diego, CA). P<0.05 was considered statistically significant.
References
Finkelman, F.D., Hogan, S.P., Hershey, G.K., Rothenberg, M.E. & Wills-Karp, M. Importance of cytokines in murine allergic airway disease and human asthma. J. Immunol. 184, 1663–1674 (2010).
Lambrecht, B.N. & Hammad, H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Ann. Rev. Immunol. 30, 243–270 (2012).
van Rijt, L.S. et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 201, 981–991 (2005).
Thornton, E.E. et al. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J. Exp. Med. 209, 1183–1199 (2012).
Brooks, C., Pearce, N. & Douwes, J. The hygiene hypothesis in allergy and asthma: an update. Curr. Opin. Allergy Clin. Immunol. 13, 70–77 (2013).
Dubois, A. et al. Regulation of Th2 responses and allergic inflammation through bystander activation of CD8+ T lymphocytes in early life. J. Immunol. 185, 884–891 (2010).
Enomoto, N. et al. Allergen-specific CTL require perforin expression to suppress allergic airway inflammation. J. Immunol. 188, 1734–1741 (2012).
Leggat, J.A. et al. Innate responsiveness of CD8 memory T-cell populations nonspecifically inhibits allergic sensitization. J. Allergy Clin. Immunol. 122, 1014–1021 (2008).
Marsland, B.J., Harris, N.L., Camberis, M., Kopf, M., Hook, S.M. & Le Gros, G. Bystander suppression of allergic airway inflammation by lung resident memory CD8+ T cells. Proc. Natl Acad. Sci. USA 101, 6116–6121 (2004).
Stock, P. et al. CD8(+) T cells regulate immune responses in a murine model of allergen-induced sensitization and airway inflammation. Eur. J. Immunol. 34, 1817–1827 (2004).
Suzuki, M., Taha, R., Ihaku, D., Hamid, Q. & Martin, J.G. CD8+ T cells modulate late allergic airway responses in Brown Norway rats. J. Immunol. 163, 5574–5581 (1999).
Takeda, K. et al. Vaccine-induced CD8+ T cell-dependent suppression of airway hyperresponsiveness and inflammation. J. Immunol. 183, 181–190 (2009).
Wells, J.W., Cowled, C.J., Giorgini, A., Kemeny, D.M. & Noble, A. Regulation of allergic airway inflammation by class I-restricted allergen presentation and CD8 T-cell infiltration. J. Allergy Clin. Immunol. 119, 226–234 (2007).
Anderson, R.J. et al. A self-adjuvanting vaccine induces cytotoxic T lymphocytes that suppress allergy. Nat. Chem. Biol. 10, 943–949 (2014).
Cohn, L., Homer, R.J., Marinov, A., Rankin, J. & Bottomly, K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J. Exp. Med. 186, 1737–1747 (1997).
Harris, N.L., Watt, V., Ronchese, F. & Le Gros, G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J. Exp. Med. 195, 317–326 (2002).
Roediger, B. et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 14, 564–573 (2013).
Plantinga, M. et al. Conventional and monocyte-derived CD11b(+) dendritic cells initiate and maintain T helper 2 cell-mediated immunity to house dust mite allergen. Immunity 38, 322–335 (2013).
Bachem, A. et al. Expression of XCR1 characterizes the Batf3-dependent lineage of dendritic cells capable of antigen cross-presentation. Front. Immunol. 3, 214 (2012).
Sung, S.S., Fu, S.M., Rose, C.E. Jr ., Gaskin, F., Ju, S.T. & Beaty, S.R. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 176, 2161–2172 (2006).
Ma, J.Z. et al. Murine CD4+ T cell responses are inhibited by cytotoxic T cell-mediated killing of dendritic cells and are restored by antigen transfer. PLoS ONE 7, e37481 (2012).
Tang, Y. et al. Antigen-specific effector CD8 T cells regulate allergic responses via IFN-gamma and dendritic cell function. J. Allergy Clin. Immunol. 129, 1611–1620 (2012).
Andrew, K.A. et al. Dendritic cells treated with lipopolysaccharide up-regulate serine protease inhibitor 6 and remain sensitive to killing by cytotoxic T lymphocytes in vivo. J. Immunol. 181, 8356–8362 (2008).
Medema, J.P. et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J. Exp. Med. 194, 657–667 (2001).
Yang, J., Huck, S.P., McHugh, R.S., Hermans, I.F. & Ronchese, F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo. Proc. Natl Acad. Sci. USA 103, 147–152 (2006).
Belz, G.T., Zhang, L., Lay, M.D., Kupresanin, F. & Davenport, M.P. Killer T cells regulate antigen presentation for early expansion of memory, but not naive, CD8+ T cell. Proc. Natl Acad. Sci. USA 104, 6341–6346 (2007).
Terrell, C.E. & Jordan, M.B. Perforin deficiency impairs a critical immunoregulatory loop involving murine CD8(+) T cells and dendritic cells. Blood 121, 5184–5191 (2013).
Lin, M.L., Zhan, Y., Villadangos, J.A. & Lew, A.M. The cell biology of cross-presentation and the role of dendritic cell subsets. Immunol. Cell Biol. 86, 353–362 (2008).
Desch, A.N. et al. Dendritic cell subsets require cis-activation for cytotoxic CD8 T-cell induction. Nat. Commun. 5, 4674 (2014).
Natsuaki, Y. et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat. Immunol. 15, 1064–1069 (2014).
Betts, R.J. & Kemeny, D.M. CD8+ T cells in asthma: friend or foe? Pharmacol. Ther. 121, 123–131 (2009).
Ege, M.J. et al. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 364, 701–709 (2011).
McMenamin, C. & Holt, P.G. The natural immune response to inhaled soluble protein antigens involves major histocompatibility complex (MHC) class I-restricted CD8+ T cell-mediated but MHC class II-restricted CD4+ T cell-dependent immune deviation resulting in selective suppression of immunoglobulin E production. J. Exp. Med. 178, 889–899 (1993).
Brimnes, M.K., Bonifaz, L., Steinman, R.M. & Moran, T.M. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J. Exp. Med. 198, 133–144 (2003).
Tsitsiou, E. et al. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J. Allergy Clin. Immunol. 129, 95–103 (2012).
Meeths, M. et al. Pathophysiology and spectrum of diseases caused by defects in lymphocyte cytotoxicity. Exp. Cell Res. 325, 10–17 (2014).
Sakai, K. & Miyazaki, J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem. Biophys. Res. Commun. 237, 318–324 (1997).
Yamazaki, C. et al. Critical roles of a dendritic cell subset expressing a chemokine receptor, XCR1. J. Immunol. 190, 6071–6082 (2013).
Harris, S.J. et al. Prediction of murine MHC class I epitopes in a major house dust mite allergen and induction of T1-type CD8+ T cell responses. Int. Immunol. 9, 273–280 (1997).
Shaw, O.M. & Harper, J.L. An efficient single prime protocol for the induction of antigen-induced airways inflammation. J. Immunol. Methods 395, 79–82 (2013).
Acknowledgements
We thank Dr William E Paul, NIH, Bethesda, USA, for gifting 4C13R mice; A/Prof Alex McLellan, University of Otago, for H2-Kbm1 BM cells, and the IMS-RCAI core facilities for ES cell screening and confocal microscopy. The expert support of the MIMR Animal Facility staff is also gratefully acknowledged. This work was funded by research grants from the Health Research Council of New Zealand to F.R., and from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to K.H., T.K., and T.O., and from a Ministry for Business Innovation and Employment New Zealand - Japan Immunology collaboration programme. N.J.D. was supported by the Rotary Club of Wellington and a PhD Scholarship from the University of Otago, New Zealand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Supplementary information
Rights and permissions
About this article
Cite this article
Daniels, N., Hyde, E., Ghosh, S. et al. Antigen-specific cytotoxic T lymphocytes target airway CD103+ and CD11b+ dendritic cells to suppress allergic inflammation. Mucosal Immunol 9, 229–239 (2016). https://doi.org/10.1038/mi.2015.55
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2015.55
This article is cited by
-
Mechanisms of CD40-dependent cDC1 licensing beyond costimulation
Nature Immunology (2022)
-
Similar immune mechanisms control experimental airway eosinophilia elicited by different allergens and treatment protocols
BMC Immunology (2019)
-
In vivo multiphoton imaging of immune cell dynamics
Pflügers Archiv - European Journal of Physiology (2016)