Abstract
The relationship between elements of the immune system and the nervous system in the presence of bacteria has been addressed recently. In particular, the sensory vanilloid receptor 1 (transient receptor potential cation channel subfamily V member 1 (TRPV1)) and the neuropeptide calcitonin gene-related peptide (CGRP) have been found to modulate cytokine response to lipopolysaccharide (LPS) independently of adaptive immunity. In this review we discuss mucosal homeostasis in the gastrointestinal tract where bacterial concentration is high. We propose that the Gram-negative bacterial receptor Toll-like receptor 4 (TLR4) can activate TRPV1 via intracellular signaling, and thereby induce the subsequent release of anti-inflammatory CGRP to maintain mucosal homeostasis.
Similar content being viewed by others
INTRODUCTION
The role of the nervous system in the development of an immune response and the effect on disease outcome is becoming a topic of interest. However, the two systems are highly complex and have very different modes of action. The nervous system is static and its functions are executed by a wide range of nerve fibers present nearly everywhere in the body. This intercalated network induces local responses through autocrine and paracrine effects of soluble factors (e.g., neurotrophins, neuropeptides, neurotransmitters), and also through cell–cell interaction. Conversely, the immune system is largely migratory in its responses using the blood, lymphatic vessels, and lymphoid organs to recruit mediators to sites of interest. Evidence has been emerging to suggest that neuronal peptides released by nerves and immune cells can play a role in regulating the immune response generated against challenge. Study of the interplay between neural and immune receptors in dealing with pathological threats may provide novel approaches to the treatment of infectious diseases.
The seminal work by Tracey and colleagues1, 2 has demonstrated a key anti-inflammatory mechanism during responses to endotoxin, termed the cholinergic anti-inflammatory pathway or inflammatory reflex. Put simply, Tracey and colleagues have shown that stimulation of the vagus nerve can downregulate tumor necrosis factor-α (TNFα) production after lipopolysaccharide (LPS) stimulation. This cross-talk is because of neural release of norepinephrine, stimulating acetycholine release from T cells and subsequent binding to the α7 nicotinic acetylcholine receptor on macrophages.3, 4 The pathway from neurotransmitter to inflammation has far reaching effects for not only understanding immune regulation,2 but also for therapeutics.5
We are particularly interested in the role of the nervous system during inflammation in the large intestine known to have vagal input in the muscularis but that also has a self-contained enteric nervous system and significant innervation by sensory fibers. Interestingly, the vagus can still affect inflammatory processes in the colon, as has been shown recently using the dextran sodium sulfate model of colitis where the vagus nerve-to-spleen axis was shown to suppress splenic CD11c+ dendritic cell activation.6 Furthermore, the vagus nerve has been shown to interact with cholinergic myenteric neurons and exert an anti-inflammatory effect on resident macrophages in small intestinal muscularis.7 However, current hypotheses regarding initiation of inflammation and regulation do not account for sensory enteric nerve activation, and yet we now know that sensory fibers themselves express receptors previously associated with immune cells (e.g., Toll-like receptor 4 (TLR4)),8, 9, 10 making them able to directly respond to inflammatory factors. It is interesting to note that TLR4 and CD14 have been found on trigeminal neurons,11 peripheral neurons,12 and neurons in the dorsal root ganglia13 alongside a range of other TLRs.13 Given that signal transduction in the nervous system is a rapid and systemic event, recognition of damage or pathogenic insult could play a significant role in induction of an immune response or indeed in homeostatic regulation of the gut micro- and macro-environments.
Ten years ago, Medzhitov and colleagues14 demonstrated that TLR signaling was essential for intestinal health, particularly during immune challenge. Although paradoxical at the time, subsequent studies have demonstrated the importance of a diverse yet “managed” microflora for intestinal homeostasis. For instance, an imbalance between bacteria from the Firmicutes phyla and enterobacteria (largely Gram negative) has repeatedly been shown for inflammatory bowel disease and T helper type 1 cell (Th1)-driven inflammation.15, 16, 17
Two molecular targets in the nervous system have been flagged for their potential role in immunity: TRPV1 (transient receptor potential cation channel subfamily V member 1), expressed on the surface of peripheral sensory fibers, and CGRP (sensory neuropeptide calcitonin gene-related peptide), released on activation of TRPV1.18 Both have been studied extensively in the context of pain induction and visceral hypersensitivity,19, 20, 21 and are also associated with immune function.22, 23 In particular, TRPV1 and CGRP have been shown to colocalize on peripheral neurons24 as well as on macrophages25, 26, 27 and dendritic cells.28 Furthermore, each has been shown to have a protective role in the large bowel.29, 30, 31
Recent years have witnessed publications addressing a potential role for bacterial recognition receptor TLR4 on TRPV1-positive nerve fibers in the oral mucosa.8, 9, 11 Studies have shown that TRPV1 is activated during chronic bacterial infection in the mouth, inducing pain but also regulating the immune response.8 Here, we will review recent literature demonstrating synergism between the sensory receptor TRPV1, the neuropeptide CGRP, and the immune response to LPS. In addition, we will discuss for the first time a proposed mechanism by which this function occurs through activation of TLR4, and how this affects not only pain induction but also homeostasis at mucosal surfaces.
TRPV1/CGRP/TLR4 AXIS
Classically, TRPV1 is expressed along the entire length of vanilloid-sensitive sensory neurons from the periphery to the somata in the central nervous system,24, 32 and this includes the sensory fibers in the large bowel. The expression of TRPV1 on immune cells has recently been described and is gaining recognition as an important mediator of immune functions.27, 33 The mechanism of TRPV1 activation/gating has been studied in detail in the past decade.28, 29, 30 Capsaicin is the most well-known exogenous agonist for TRPV1; however, neuronal TRPV1 is activated by a range of additional endogenous agonists such as heat (>43 °C),34, 35 protons (∼pH 4.5),36, 37 voltage,38 and prostaglandin E2,37 and lipids such as anandamide39 and phosphatidylinositol(4,5)-biphosphate.40 All activate TRPV1 by distinct molecular recognition sites36 (Figure 1) and this induced signaling triggers CGRP release, demonstrating that TRPV1+ fibers and immune cells are able to respond directly to an inflammatory milieu.
TRPV1 is predicted to have six transmembrane domains with a proposed short, pore-forming hydrophilic stretch region between the fifth and sixth transmembrane domain (P-loop). Like many TRP channels, TRPV1 has a long cytoplasmic N-terminal (containing three ankyrin-repeat domains) and C-terminal (containing a TRP domain close to the sixth transmembrane domains). The N-terminus92 and C-terminus residues, Arg114 and Glu761, respectively, are vanilloid recognition sites93 that vary between species94 and different stimuli have different recognition sites on a single receptor.34 S-502: PKA-mediated phosphorylation; S-502 and S-800: PKC-mediated phosphorylation (modified from Lu et al.95 and Pingle et al.96). PIP2, phosphatidylinositol(4,5)-biphosphate; PKA, protein kinase A; PKC, protein kinase C; TRPV1, transient receptor potential cation channel subfamily V member 1.
Recent studies have shown that LPS can modulate TRPV1 function. This is important in the context of gut health because CGRP, the downstream effector of TRPV1 activation, has been shown to be anti-inflammatory in the large bowel.29, 30, 31, 41 CGRP receptor is found on immune cells. Consequently, CGRP can downregulate TNFα in macrophages,42 osteoclasts,43 and dendritic cells.44 Furthermore, this anti-inflammatory effect is specific for TNFα; CGRP binding to its receptor causes rapid upregulation of inducible cyclic adenosine monophosphate early repressor (ICER), suppressing TNFα transcription.44, 45
Clearly, the presence of “healthy” flora demands a level of tolerance. However, a healthy gut also needs to be able to detect changes in phyla, not only to signal pathological threat but also to initiate regulatory mechanisms. Recent data have suggested that elevated levels of Gram-negative bacteria are associated with small and large bowel inflammation.46 It is known that the balance of Firmicutes and Bacteroidetes phyla in the large bowel can have significant consequences for gut homeostasis and response to inflammatory challenge.
MODULATION OF TRPV1 FUNCTION BY LPS IN VITRO
The response of a calcium channel (such as TRPV1) to ligand binding carries a threshold for activation where commitment to signaling is achieved above certain limits, as in synaptic activation. Here we discuss independent studies that complement each other with respect to TRPV1 activation by LPS. LPS alone is unable to activate TRPV1 in vitro. For instance, Ferraz et al.9 incubated LPS from Prophyromona gingivalis at 2 μg ml−1 in vitro with cultured trigeminal neurons. They were interested in whether there was a link between the presence of P. gingivalis in the oral cavity and the sensation of pain during bacterial infection. Indeed, trigeminal neurons pretreated with LPS produced a significant increase in CGRP release when treated with capsaicin, a natural ligand for TRPV1, suggesting a lower threshold of activation for TRPV1 during infection. The same group identified via confocal microscopy, the colocalization of TLR4 and TRPV1 on CGRP-positive neurons in the tooth pulp and suggested that enhanced TRPV1 activation may occur through TLR4 activation on the same neurons. Finally, Chung et al.47 showed that LPS administered to freshly cut dentine could upregulate TRPV1 expression significantly 1 day after treatment, associating this observation with the early onset of pain seen during bacterial infections in the oral cavity. Importantly, in all these studies, LPS could not induce ligand-free TRPV1 activation and CGRP release. Inhibition of TLR4 resulted in a complete reversal of all observations, clearly linking the LPS/TLR4 interaction to the LPS/TRPV1 sensitization observations.
MODULATION OF TRPV1 FUNCTION BY LPS IN VIVO
TRPV1 has been shown to protect against endotoxemia induced by LPS. Clark et al.10 administered LPS (11.25 million EU per kg in saline) to TRPV1-deficient mice in order to investigate the role of TRPV1 in the onset of various pathological components of systemic endotoxemia. TRPV1-deficient mice expressed a rapid drop in blood pressure 1 to 2 h after LPS because of high nitric oxide levels in the blood. In addition, TNFα levels were elevated in the peritoneum, attributed to the activation of macrophages after LPS recognition and TLR4 signaling.48 TRPV1-deficient mice also had liver failure, directly attributable to the absence of CGRP.49 Similarly, Tsuji et al.50 demonstrated that LPS (30 mg kg−1) induced CGRP in the serum over and above those observed with exogenous TRPV1 ligand capsaicin. As it has been shown that CGRP can inhibit macrophage function by inhibiting TNFα release through the increase in cyclic adenosine monophosphate,42 the authors concluded that this increase in CGRP levels was protective through the control of serum levels of TNFα.50 These studies have demonstrated a clear regulatory role for TRPV1 activation and, by implication, CGRP.
The cecal ligation puncture model is used as a model for sepsis, often inducing a fatal inflammatory response. However, the precise amount of bacteria released after puncture is variable. Fernandes et al.51 investigated the effect of the deletion of TRPV1 on the onset of the systemic inflammatory response syndrome to sepsis using the cecal ligation puncture model. As expected from data in previous studies, TRPV1-deficient mice had hypothermia, hypotension, and liver dysfunction compared with wild-type mice. This was associated with increased levels of inflammatory mediators TNFα, nitric oxide, and interleukin (IL)-1β (which have been associated with hyperalgesia52) and the immune regulators IL-10 and IL-6. All the pro-inflammatory elevations in deficient mice could be attributed to the absence of CGRP.
CGRP MODULATION OF LPS-INDUCED IMMUNE RESPONSE
CGRP is a well-known potent vasodilator (nerve-mediated) and Th2 cytokine inducer (cell mediated).53, 54 Although CGRP is often used as a TRPV1 activation marker, it has a key role in the inflammatory response to LPS.42, 55 In particular, CGRP has been shown to exert either pro- or anti- inflammatory effects, dependent on concentration,56 through the actions of TNFα55 and/or IL-10.45
Gomes et al.57 examined the effect of CGRP on local and systemic acute inflammation in a peritoneal model of sepsis. In acute inflammation, mice pretreated with four different doses of CGRP (1 × 10−6 −1 μg per cavity) 30 min before Escherichia coli LPS (250 ng per cavity) showed a dose-dependent decrease in neutrophil accumulation in the peritoneum and blood 4 h after treatment. CGRP was able to inhibit keratinocyte chemoattractant levels 90 min after LPS administration; keratinocyte chemoattractant is involved in chemotaxis and activation of neutrophils. All observations were reversed with the CGRP receptor antagonist hCGRP 8-37. These results clearly demonstrated the ability of CGRP to modulate an acute inflammatory response to LPS by controlling neutrophil recruitment.
Gomes et al.57 also examined the effect of CGRP given before LPS (500 μg per cavity), using the same relatively small dose of CGRP (1 μg per cavity) as previously described. They found the neuropeptide to be protective against LPS-driven inflammation, downregulating TNFα while significantly upregulating IL-10.57 CGRP also reduced the rate of mortality by 80% compared with LPS alone.
Similarly, the secretion of CGRP from macrophages was demonstrated by Ma et al.56 who showed a similar dose response to LPS as that shown for neurons; macrophages produced the highest amount of CGRP after exposure to an LPS dose of 1 μg/ml, but were unable to secrete CGRP after exposure to low-dose LPS. In other words, a threshold for activation of TLR4 had to be met by macrophages to release CGRP. There is therefore a definite correlation between LPS, the activation/upregulation of TRPV1, and the subsequent release of CGRP and modulation of the immune response through TNFα. TRPV1 seems to play a critical role in dampening the immune response and aiding host protection through this mechanism.
It is worth noting that although CGRP is a key regulatory neuropeptide with important roles in immune outcome, substance P has also emerged as a dominant promoter of pro-inflammatory function.30 Numerous reports show opposing effects of CGRP and substance P in models of colitis.58, 59, 60, 61, 62 Therefore, it is widely understood that although CGRP exerts a regulatory role on LPS-induced inflammatory mediators such as TNFα, the regulation of CGRP itself is more likely controlled via the neuropeptide substance P.
ROLE OF TRPV1/CGRP IN GUT HOMEOSTASIS
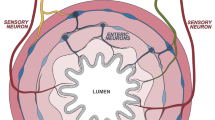
It is clear from the studies outlined above that TRPV1 signaling can have a dramatic effect on immune responses to bacteria, but how and why is this relevant to mucosal homeostasis? The gut is the second most innervated organ in the body, and TRPV1+/CGRP+ fibers are abundant.24 Nerve fibers have the potential to respond rapidly to environmental stimuli, including commensal bacteria. The fact that secreted neuropeptides such as CGRP are able to interact with cells of the immune system demonstrates the potential for an integrated physiology to maintain mucosal health. It is well established that dendritic cells sit in very close proximity to TRPV1+ neurons and that CGRP can downregulate antigen presentation and migration of dendritic cells in both the skin54, 63 and lung.64 Furthermore, CGRP has been shown to directly downregulate dendritic cell responses to TLR ligands.44 These studies suggest a host response to LPS that induces regulation; activation of TLR4 in the presence of TRPV1 ligands sensitizes TRPV1, lowering the threshold for activation, inducing release of CGRP, and promoting subsequent anti-inflammatory effects.65 CGRP therefore has the potential to provide host protection without compromising protective immune response (Figure 2).
LPS sensitizes the TLR4/TRPV1/CGRP pathway. Activation of TLR4 on macrophages and sensory nerves subsequently activates TRPV1 releasing CGRP.18 Anti-inflammatory effects of CGRP include indirect modulation of TNFα transcription via binding to CGRP receptor and upregulation of ICER (dotted line). LPS is shown as red dots. CGRP, sensory neuropeptide calcitonin gene-related peptide; ICER, inducible cyclic adenosine monophosphate early repressor; LPS, lipopolysaccharide; TLR4, Toll-like receptor 4; TNFα, tumor necrosis factor-α; TRPV1, transient receptor potential cation channel subfamily V member 1. A full color version of this figure is available at the Mucosal Immunology journal online.
ALTERNATIVE TRP CHANNELS AS A SOURCE OF CGRP
Interestingly, TRPV1 is functionally linked with another member of the TRP family, the transient receptor potential ankyrin 1 (TRPA1), that responds to noxious cold66 and is also expressed on nociceptive sensory neurons. TRPA1 and TRPV1 have been shown to form a heteromer assembly (a feature common between members of TRP family) and it appears that both receptors share some physiological characteristics and influence one another.67 In particular, TRPA1 has been identified as a receptor that can respond to LPS, although independently of TLR4.68 Similar to TRPV1 activation, receptor signaling results in CGRP and substance P release,30, 59, 69 although through an alternative mechanism. The involvement of TRPA1 in immune functions has been demonstrated recently in studies of colitis.23, 70, 71, 72 Both TRPV1 and TRPA1 are emerging as key components of the neuroimmune regulatory/inflammatory processes during gut inflammation, centered around the induction of CGRP.
A CRUCIAL ROLE FOR PKCɛ IN THE ACTIVATION OF TRPV1 VIA TLR4
The observations so far discussed are highly suggestive of an intracellular cross-talk between TLR4 and TRPV1 on nerves and immune cells. However, the molecular pathway responsible for TRPV1 sensitization as a result of TLR signaling is not defined, despite a clear correlation between sepsis and pain induction.8, 9, 10
There are many candidate intracellular families that link the two receptors together functionally, one of which is the phospholipid-dependent serine/threonine kinase family, a key component in intracellular signaling cascades. For example, protein kinase A (PKA) has been reported active in respect of calcium channels.70, 71 TRPV1 agonists are known to trigger the synthesis of cyclic adenosine monophosphate by adenylyl cyclase; for example, prostaglandin E2 activates PKA that sensitizes TRPV1 as a result. PKA phosphorylation-binding sites on TRPV1 are S6, S116, T144, T370, S502, S774, and S820.72 These form part of the large six ankyrin repeats that form part of the later N terminus of TRPV1. Phosphorylation of these binding sites have implicated PKA in heat and hyperalgesia sensitization and desensitization through TRPV1 depending on where it binds.28, 73, 74 Interestingly, PKA binding to TRPA1 was indirectly capable of activating TRPV1 via S116,75 showing an intriguing correlation between the two receptors in this manner. No studies as yet have shown whether PKA alone can activate TRPV1 independently of any agonists. However, recently a scaffolding PKA protein known as AKAP79/150 was suggested to play a critical role in the phosphorylation process of TRPV1 by PKA.76 These studies and others highlight the importance of PKA in the regulation and sensitization of TRPV1.
However, one of the strongest candidates of the protein kinase family is protein kinase C (PKC). PKC is not only activated after LPS binding to TLR4 but is also known for its many roles in TRPV1 sensitization/activation.77, 78 Different PKCs develop at various developmental stages starting from birth, and are also expressed in different areas throughout the central nervous system.79 The immunologically active nPKCɛ has been found in the forebrain, the spinal cord, and dorsal root ganglia sensory neurons.80 In addition, PKCs are associated with TRPV1.81 It is well known that TRPV1 ion channels have a range of phosphorylation sites for many agonists (Figure 1). Two of these binding sites are PKC specific at S502 and S800,82 and nPKCɛ is the main isoform for these binding sites,79 confirmed by crystallography.83 Relative binding sites for PKC on TRPA1 have not yet been identified. Interestingly, some studies have suggested that PKC isoforms can activate TRPV1 without the need of any further agonists.78, 84, 85 For instance, Bonnington et al.77 found that nerve growth factor utilizes PKC in order to activate TRPV1 and enhance its sensitivity to capsaicin by 37%. Ferreira et al.86 demonstrated that the PKC activator phorbol 12-myristate 13-acetate was able to prolong nociception in the mouse paw (site of infection), implicating prolonged TRPV1 activation.
LPS binding to TLR4 also results in the activation of PKC in immune cells.87, 88, 89 LPS inducible IL1-receptor associated kinase-2 (IRAK2) activation can be inhibited by a PKCζ inhibitor calphostin,90 whereas LPS activation of dendritic cells and macrophages results in nPKCɛ activation,87 a response essential to Th1 cytokine induction.88 These observations were confirmed with a PKC isoform inhibitor able to diminish the production of interferon-γ and IL-12 in response to LPS,87 thus demonstrating that nPKCɛ plays a critical role in nuclear factor-κB and mitogen-activated protein kinase activation and functions as a key player in cytokine production in response to an LPS challenge.87, 88
It is distinctly possible that TLR4-activated PKC could play a part in the function of TRPV1+ fibers, providing natural regulatory feedback on exposure to bacteria. PKC activation by TLR4 could activate/sensitize TRPV1 via its phosphorylation-binding sites. At one level, this would result in a homeostatic local response by the cell responding to LPS, whether a macrophage or neuron, as illustrated in Figure 3.
LPS activates TLR4 that in turn initiates an intracellular cascade (TIRAP, MyD88, IRAK2, TRAF6) leading to the downstream activation of NF-κB and MAPK and the production of cytokines. LPS additionally activates PKCɛ through TIRAP and MyD88 that can potentially bind to TRPV1 via S800- and S502-binding sites. This results in the release of the modulatory neuropeptide CGRP.77 Other protein kinases have the ability to bind to TRPV1, i.e., PKA. In addition, Ca2+ (recruited through intracellular influx as a result of natural ligand binding to TRPV1) can activate PKCɛ for further modulation. CGRP, sensory neuropeptide calcitonin gene-related peptide; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; PKA, protein kinase A; PKC, protein kinase C; TLR4, Toll-like receptor 4; TRPV1, transient receptor potential cation channel subfamily V member 1.
SUMMARY
TRPV1 and CGRP have already been shown to have a protective role in the large bowel during chronic inflammation.29, 30, 31 We know that reduced sensory and motor function and reduced responses to TRPV1 ligands are also associated with chronic gut inflammation.91 It is clear that sensory receptors on many immune cells can contribute to immune responses. However, here we have outlined in particular an enteric neural mechanism for CGRP induction, aided by gut flora and TLR4 signaling, providing a local regulatory component to “normal” LPS responses.91 It would be foolish to suggest that the primary role of the enteric nervous system is homeostatic balance, but it is clear that both sensory fibers and immune cells have the ability to regulate the response to bacteria via TRPV1, independent of both neurotransmitters and an adaptive immune response. We propose that TRPV1+-CGRP+ sensory fibers form an integral part of gut homeostasis, and their absence (in the case of inflammatory neuropathy) or dysfunction (in the absence of stimulating microflora) leads to the perpetuation of gut inflammation. Understanding more about the interplay of microflora and the nervous system in the context of immune function will undoubtedly further our understanding of mucosal biology and inflammation.
References
Borovikova, L.V. et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 (2000).
Pavlov, V.A., Wang, H., Czura, C.J., Friedman, S.G. & Tracey, K.J. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 9, 125–134 (2003).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 (2011).
Wang, H. et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388 (2003).
Olofsson, P.S., Rosas-Ballina, M., Levine, Y.A. & Tracey, K.J. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol. Rev. 248, 188–204 (2012).
Ji, H. et al. Central cholinergic activation of a vagus nerve-to-spleen circuit alleviates experimental colitis. Mucosal Immunol. 7, 335–347 (2014).
Matteoli, G. et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63, 938–948 (2014).
Diogenes, A., Ferraz, C.C., Akopian, A.N., Henry, M.A. & Hargreaves, K.M. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J. Dent. Res. 90, 759–764 (2011).
Ferraz, C.C., Henry, M.A., Hargreaves, K.M. & Diogenes, A. Lipopolysaccharide from Porphyromonas gingivalis sensitizes capsaicin-sensitive nociceptors. J. Endod. 37, 45–48 (2011).
Clark, N. et al. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J. 21, 3747–3755 (2007).
Wadachi, R. & Hargreaves, K.M. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J. Dent. Res. 85, 49–53 (2006).
Goethals, S., Ydens, E., Timmerman, V. & Janssens, S. Toll-like receptor expression in the peripheral nerve. Glia 58, 1701–1709 (2010).
Qi, J. et al. Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J. Immunol. 186, 6417–6426 (2011).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Friswell, M., Campbell, B. & Rhodes, J. The role of bacteria in the pathogenesis of inflammatory bowel disease. Gut Liver 4, 295–306 (2010).
Smits, H.H. et al. Commensal Gram-negative bacteria prime human dendritic cells for enhanced IL-23 and IL-27 expression and enhanced Th1 development. Eur. J. Immunol. 34, 1371–1380 (2004).
Sokol, H. & Seksik, P. The intestinal microbiota in inflammatory bowel diseases: time to connect with the host. Curr. Opin. Gastroenterol. 26, 327–331 (2010).
Meng, J. et al. Activation of TRPV1 mediates calcitonin gene-related peptide release, which excites trigeminal sensory neurons and is attenuated by a retargeted botulinum toxin with anti-nociceptive potential. J. Neurosci. 29, 4981–4992 (2009).
Akbar, A. et al. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 59, 767–774 (2010).
Vermeulen, W. et al. Role of TRPV1 and TRPA1 in visceral hypersensitivity to colorectal distension during experimental colitis in rats. Eur. J. Pharmacol. 698, 404–412 (2013).
Zhu, H. et al. Interaction between protein kinase D1 and transient receptor potential V1 in primary sensory neurons is involved in heat hypersensitivity. Pain 137, 574–588 (2008).
Hughes, P.A. et al. TRPV1-expressing sensory fibers and IBS: links with immune function. Gut 58, 465–466 (2009).
Levite, M. Nerve-driven immunity. The direct effects of neurotransmitters on T-cell function. Ann. NY Acad. Sci. 917, 307–321 (2000).
Matsumoto, K. et al. Distribution of transient receptor potential vanilloid 1 channel-expressing nerve fibers in mouse rectal and colonic enteric nervous system: relationship to peptidergic and nitrergic neurons. Neuroscience 172, 518–534 (2011).
Linscheid, P. et al. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit. Care Med. 32, 1715–1721 (2004).
Fernandez, S., Knopf, M.A., Bjork, S.K. & McGillis, J.P. Bone marrow-derived macrophages express functional CGRP receptors and respond to CGRP by increasing transcription of c-fos and IL-6 mRNA. Cell. Immunol. 209, 140–148 (2001).
Zhao, J.F. et al. Activation of TRPV1 prevents OxLDL-induced lipid accumulation and TNF-alpha-induced inflammation in macrophages: role of liver X receptor alpha. Mediators Inflamm. 2013, 925171 (2013).
Toth, B.I. et al. Transient receptor potential vanilloid-1 signaling inhibits differentiation and activation of human dendritic cells. FEBS Lett. 583, 1619–1624 (2009).
Engel, M.A. et al. The proximodistal aggravation of colitis depends on substance P released from TRPV1-expressing sensory neurons. J. Gastroenterol. 47, 256–265 (2012).
Engel, M.A. et al. Opposite effects of substance P and calcitonin gene-related peptide in oxazolone colitis. Dig. Liver Dis. 44, 24–29 (2011).
Lee, J., Yamamoto, T., Kuramoto, H. & Kadowaki, M. TRPV1 expressing extrinsic primary sensory neurons play a protective role in mouse oxazolone-induced colitis. Auton. Neurosci. 166, 72–76 (2012).
Szallasi, A. Autoradiographic visualization and pharmacological characterization of vanilloid (capsaicin) receptors in several species, including man. Acta Physiol. Scand. Suppl. 629, 1–68 (1995).
Basu, S. & Srivastava, P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc. Natl. Acad. Sci. USA 102, 5120–5125 (2005).
Caterina, M.J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824 (1997).
Tominaga, M. et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543 (1998).
Jordt, S.E., Tominaga, M. & Julius, D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. USA 97, 8134–8139 (2000).
Vyklicky, L. et al. Inflammatory mediators at acidic pH activate capsaicin receptors in cultured sensory neurons from newborn rats. J. Neurophysiol. 79, 670–676 (1998).
Gunthorpe, M.J., Harries, M.H., Prinjha, R.K., Davis, J.B. & Randall, A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1). J. Physiol. 525 (Pt 3), 747–759 (2000).
Olah, Z., Karai, L. & Iadarola, M.J. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J. Biol. Chem. 276, 31163–31170 (2001).
Chuang, H.H. et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411, 957–962 (2001).
Szitter, I. et al. The role of transient receptor potential vanilloid 1 (TRPV1) receptors in dextran sulfate-induced colitis in mice. J. Mol. Neurosci. 42, 80–88 (2010).
Feng, Y., Tang, Y., Guo, J. & Wang, X. Inhibition of LPS-induced TNF-alpha production by calcitonin gene-related peptide (CGRP) in cultured mouse peritoneal macrophages. Life Sci. 61 PL 281–287 (1997).
Millet, I. & Vignery, A. The neuropeptide calcitonin gene-related peptide inhibits TNF-alpha but poorly induces IL-6 production by fetal rat osteoblasts. Cytokine 9, 999–1007 (1997).
Altmayr, F., Jusek, G. & Holzmann, B. The neuropeptide calcitonin gene-related peptide causes repression of tumor necrosis factor-alpha transcription and suppression of ATF-2 promoter recruitment in Toll-like receptor-stimulated dendritic cells. J. Biol. Chem. 285, 3525–3531 (2010).
Harzenetter, M.D. et al. Negative regulation of TLR responses by the neuropeptide CGRP is mediated by the transcriptional repressor ICER. J. Immunol. 179, 607–615 (2007).
Im, E., Riegler, F.M., Pothoulakis, C. & Rhee, S.H. Elevated lipopolysaccharide in the colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G490–G497 (2012).
Chung, M.K., Lee, J., Duraes, G. & Ro, J.Y. Lipopolysaccharide-induced pulpitis up-regulates TRPV1 in trigeminal ganglia. J. Dent. Res. 90, 1103–1107 (2011).
Cunha, F.Q. et al. Differential induction of nitric oxide synthase in various organs of the mouse during endotoxaemia: role of TNF-alpha and IL-1-beta. Immunology 81, 211–215 (1994).
Harada, N., Okajima, K., Uchiba, M., Kurihara, H. & Nakagata, N. Antithrombin reduces reperfusion-induced liver injury in mice by enhancing sensory neuron activation. Thromb. Haemost. 95, 788–795 (2006).
Tsuji, F. et al. Transient receptor potential vanilloid 1 agonists as candidates for anti-inflammatory and immunomodulatory agents. Eur. J. Pharmacol. 627, 332–339 (2010).
Fernandes, E.S. et al. TRPV1 deletion enhances local inflammation and accelerates the onset of systemic inflammatory response syndrome. J. Immunol. 188, 5741–5751 (2012).
Morgan, M.M., Clayton, C.C. & Heinricher, M.M. Dissociation of hyperalgesia from fever following intracerebroventricular administration of interleukin-1beta in the rat. Brain Res. 1022, 96–100 (2004).
Smillie, S.J. & Brain, S.D. Calcitonin gene-related peptide (CGRP) and its role in hypertension. Neuropeptides 45, 93–104 (2011).
Ding, W., Stohl, L.L., Wagner, J.A. & Granstein, R.D. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J. Immunol. 181, 6020–6026 (2008).
Jusek, G., Reim, D., Tsujikawa, K. & Holzmann, B. Deficiency of the CGRP receptor component RAMP1 attenuates immunosuppression during the early phase of septic peritonitis. Immunobiology 217, 761–767 (2012).
Ma, W., Dumont, Y., Vercauteren, F. & Quirion, R. Lipopolysaccharide induces calcitonin gene-related peptide in the RAW264.7 macrophage cell line. Immunology 130, 399–409 (2010).
Gomes, R.N. et al. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock 24, 590–594 (2005).
Mazelin, L., Theodorou, V., Fioramonti, J. & Bueno, L. Vagally dependent protective action of calcitonin gene-related peptide on colitis. Peptides 20, 1367–1374 (1999).
Stucchi, A.F. et al. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 279, G1298–G1306 (2000).
Evangelista, S. & Tramontana, M. Involvement of calcitonin gene-related peptide in rat experimental colitis. J. Physiol. Paris 87, 277–280 (1993).
Di Sebastiano, P. et al. SR140333, a substance P receptor antagonist, influences morphological and motor changes in rat experimental colitis. Dig. Dis. Sci. 44, 439–444 (1999).
Mazelin, L. et al. Comparative effects of nonpeptide tachykinin receptor antagonists on experimental gut inflammation in rats and guinea-pigs. Life Sci. 63, 293–304 (1998).
Mikami, N. et al. Calcitonin gene-related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J. Immunol. 186, 6886–6893 (2011).
Rochlitzer, S. et al. The neuropeptide calcitonin gene-related peptide affects allergic airway inflammation by modulating dendritic cell function. Clin. Exp. Allergy 41, 1609–1621 (2011).
Holzmann, B. Antiinflammatory activities of CGRP modulating innate immune responses in health and disease. Curr. Protein Pept. Sci. 14, 268–274 (2013).
Bandell, M. et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 (2004).
Fischer, M.J. et al. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch. PMID: 24643480 (e-pub ahead of print).
Meseguer, V. et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 5, 3125 (2014).
Engel, M.A. et al. TRPA1 and substance P mediate colitis in mice. Gastroenterology 141, 1346–1358 (2011).
Epstein, D.J., Marti, E., Scott, M.P. & McMahon, A.P. Antagonizing cAMP-dependent protein kinase A in the dorsal CNS activates a conserved Sonic hedgehog signaling pathway. Development 122, 2885–2894 (1996).
Brown, J.A., Diggs-Andrews, K.A., Gianino, S.M. & Gutmann, D.H. Neurofibromatosis-1 heterozygosity impairs CNS neuronal morphology in a cAMP/PKA/ROCK-dependent manner. Mol. Cell. Neurosci. 49, 13–22 (2012).
Spahn, V. et al. Opioid withdrawal increases transient receptor potential vanilloid 1 activity in a protein kinase A-dependent manner. Pain 154, 598–608 (2013).
Rathee, P.K. et al. PKA/AKAP/VR-1 module: a common link of Gs-mediated signaling to thermal hyperalgesia. J. Neurosci. 22, 4740–4745 (2002).
Mohapatra, D.P. & Nau, C. Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J. Biol. Chem. 278, 50080–50090 (2003).
Spahn, V., Stein, C. & Zollner, C. Modulation of transient receptor vanilloid 1 activity by transient receptor potential ankyrin 1. Mol. Pharmacol. 85, 335–344 (2014).
Efendiev, R., Bavencoffe, A., Hu, H., Zhu, M.X. & Dessauer, C.W. Scaffolding by A-kinase anchoring protein enhances functional coupling between adenylyl cyclase and TRPV1 channel. J. Biol. Chem. 288, 3929–3937 (2013).
Bonnington, J.K. & McNaughton, P.A. Signalling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J. Physiol. 551, 433–446 (2003).
Premkumar, L.S. & Ahern, G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature 408, 985–990 (2000).
Mandadi, S., Armati, P.J. & Roufogalis, B.D. Protein kinase C modulation of thermo-sensitive transient receptor potential channels: implications for pain signaling. J. Nat. Sci. Biol. Med. 2, 13–25 (2012).
Saito, N. et al. Cellular and intracellular localization of epsilon-subspecies of protein kinase C in the rat brain; presynaptic localization of the epsilon-subspecies. Brain Res. 607, 241–248 (1993).
Velazquez, K.T., Mohammad, H. & Sweitzer, S.M. Protein kinase C in pain: involvement of multiple isoforms. Pharmacol. Res. 55, 578–589 (2007).
Tominaga, M. & Tominaga, T. Structure and function of TRPV1. Pflugers Arch. 451, 143–150 (2005).
Liao, M., Cao, E., Julius, D. & Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112 (2013).
Millan, M.J. The induction of pain: an integrative review. Prog. Neurobiol. 57, 1–164 (1999).
Numazaki, M., Tominaga, T., Toyooka, H. & Tominaga, M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J. Biol. Chem. 277, 13375–13378 (2002).
Ferreira, J., Triches, K.M., Medeiros, R. & Calixto, J.B. Mechanisms involved in the nociception produced by peripheral protein kinase c activation in mice. Pain 117, 171–181 (2005).
Aksoy, E., Amraoui, Z., Goriely, S., Goldman, M. & Willems, F. Critical role of protein kinase C epsilon for lipopolysaccharide-induced IL-12 synthesis in monocyte-derived dendritic cells. Eur. J. Immunol. 32, 3040–3049 (2002).
Castrillo, A. et al. Protein kinase Cepsilon is required for macrophage activation and defense against bacterial infection. J. Exp. Med. 194, 1231–1242 (2001).
Devadas, K., Hewlett, I.K. & Dhawan, S. Lipopolysaccharide suppresses HIV-1 replication in human monocytes by protein kinase C-dependent heme oxygenase-1 induction. J. Leukoc. Biol. 87, 915–924 (2010).
Hu, J., Jacinto, R., McCall, C. & Li, L. Regulation of IL-1 receptor-associated kinases by lipopolysaccharide. J. Immunol. 168, 3910–3914 (2002).
Smith, A.S. & Smid, S.D. Impaired capsaicin and neurokinin-evoked colonic motility in inflammatory bowel disease. J. Gastroenterol. Hepatol. 20, 697–704 (2005).
Kuzhikandathil, E.V. et al. Functional analysis of capsaicin receptor (vanilloid receptor subtype 1) multimerization and agonist responsiveness using a dominant negative mutation. J. Neurosci. 21, 8697–8706 (2001).
Jung, J. et al. Agonist recognition sites in the cytosolic tails of vanilloid receptor 1. J. Biol. Chem. 277, 44448–44454 (2002).
Gavva, N.R. et al. Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 279, 20283–20295 (2004).
Lu, I.J., Lee, K.Z., Lin, J.T. & Hwang, J.C. Capsaicin administration inhibits the abducent branch but excites the thyroarytenoid branch of the recurrent laryngeal nerves in the rat. J. Appl. Physiol. 98, 1646–1652 (2005).
Pingle, S.C., Matta, J.A. & Ahern, G.P. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb. Exp. Pharmacol. 179, 155–171 (2007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Rights and permissions
This work is licensed under the Creative Commons Attribution-NonCommercial-No Derivative Works 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Assas, B., Miyan, J. & Pennock, J. Cross-talk between neural and immune receptors provides a potential mechanism of homeostatic regulation in the gut mucosa. Mucosal Immunol 7, 1283–1289 (2014). https://doi.org/10.1038/mi.2014.80
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2014.80
This article is cited by
-
CGRP Monoclonal Antibodies for Migraine: Rationale and Progress
BioDrugs (2017)
-
“TRP inflammation” relationship in cardiovascular system
Seminars in Immunopathology (2016)
-
Capsaicin for Rhinitis
Current Allergy and Asthma Reports (2016)
-
Ebola: translational science considerations
Journal of Translational Medicine (2015)