Abstract
Although regulatory T cells (Tregs) have been implicated in inflammatory bowel disease, Tregs from Crohn's disease (CD) patients are increased in number and function normally in vitro. To clarify this disparity, we studied Treg function in vivo using a spontaneous model of CD-like ileitis. We first administered anti-CD25-depleting antibodies to SAMP1/YitFc (SAMP) mice to assess ileitis; mesenteric lymph node cells were then transferred into SCID (severe combined immunodeficient) recipients to induce colitis. CD25 depletion increased the severity of both spontaneous ileitis and adoptively transferred colitis. Interestingly, a second transfer of CD4+CD25+ cells from untreated AKR control mice was able to ameliorate the induced colitis, whereas CD4+CD25+ cells from untreated SAMP mice were not, suggesting a functional abnormality in SAMP Tregs. Anti-CD25 treatment in SAMP mice also induced proliferation of CD25−Foxp3+ Tregs, which had a proinflammatory intestinal T helper type 1/ T helper type 2 (Th1/Th2) effector phenotype. These studies demonstrate Treg dysfunction in a spontaneous model of CD-like ileitis.
Similar content being viewed by others
INTRODUCTION
CD4+CD25+Foxp3+ natural regulatory T cells (Tregs), which comprise 5–10% of CD4+ T cells in naive mice, have a potent regulatory capacity and may play an important role in several autoimmune and infectious diseases, including Crohn's disease (CD).1, 2, 3, 4, 5 The intestinal immune system orchestrates a complex balance between proinflammatory and anti-inflammatory responses to luminal antigens; any disruptions to this balance may give rise to intestinal inflammation. Adoptive transfer studies in animal models of colitis have demonstrated a critical role of Tregs in regulating chronic intestinal inflammation.6, 7 However, the number of Tregs are expanded in the mucosal lymphoid tissues in CD patients, and cultured Tregs isolated from the colonic mucosa of CD patients display normal functional properties when assessed under in vitro conditions.8, 9 Thus, it remains unclear whether Treg dysfunction contributes to the pathogenesis of CD. Few mechanistic studies have been performed to assess the function of Tregs in vivo.
We have extensively characterized the SAMP1/YitFc (SAMP) spontaneous mouse model of ileitis, which has many similarities to CD in humans.10, 11 Similar to CD, inflammation in SAMP mice is primarily localized to the terminal ileum with active and chronic histological features that include transmural, discontinuous, and granulomatous inflammation. In addition, a subset of mice develops perianal disease. SAMP ileitis responds to administration of common CD treatments, including corticosteroids and anti-tumor necrosis factor (anti-TNF) antibodies.12 The initiation of SAMP ileitis involves an induction phase that precedes the histological injury and shows the characteristics of a T helper type 1 (Th1)-mediated condition,13 whereas the maintenance phase that follows the development of chronic ileitis displays a mixed Th1/Th2 phenotype.14 SAMP ileitis is mediated by CD4+ cells, which have a decreased Treg frequency when raised under germ-free conditions.15 However, the specific in vivo function of Tregs during spontaneous CD-like ileitis in SAMP mice remains unclear.
In this report, we used anti-CD25 antibodies (Abs), a well-established method to deplete natural Tregs in vivo, to elucidate their functional role in a spontaneous murine model of CD. We describe three novel findings: (i) in vivo depletion of SAMP Tregs significantly increased the severity of spontaneous ileitis; transfer of Treg-depleted SAMP mesenteric lymph node (MLN) cells significantly increased the severity of adoptively transferred SAMP colitis, (ii) a second transfer of non-depleted CD4+CD25+ cells isolated from AKR control mice was able to ameliorate the adoptively transferred SAMP colitis; transfer of nondepleted cells from SAMP mice failed to do so, and (iii) CD25−Foxp3+ cells, which were significantly expanded in SAMP mice following Treg depletion, do not possess a regulatory function and appear to have a colitogenic phenotype. To our knowledge, these observations provide the first direct in vivo evidence suggesting that Tregs are dysfunctional in a spontaneous model of CD-like intestinal inflammation.
RESULTS
Anti-CD25 Ab treatment depletes CD25+ cells, but not Foxp3+ cells, in SAMP mice
We first tested the extent of Treg depletion after 6 weeks of anti-CD25 treatment. In the MLNs of SAMP and AKR mice, 99.5% of CD25+ cells were eliminated following anti-CD25 Ab treatment using either Clone PC61 (Figure 1a) or Clone 3C7, a noncompeting anti-CD25 monoclonal antibody (mAb; Supplementary Figure S1 online). In the spleens, CD25+ cells were also completely eliminated (99.9%, data not shown). In MLN cells of untreated SAMP mice, the proportion of CD4+Foxp3+ cells was significantly higher than in untreated AKR mice (SAMP: 12.5±0.7% vs. AKR: 9.8±0.3%, P=0.012, Figure 1b), and the total number of CD4+Foxp3+ cells in SAMP MLNs was 3–5 times more than that observed in AKR MLNs. To our surprise, we found that only 28.3% of Foxp3+ cells in the MLNs of SAMP mice were eliminated following anti-CD25 treatment, compared with 72.0% of Foxp3+ cells in AKR control mice (Figure 1b). The same trend was also observed in the spleen (SAMP: 28.5% reduction, AKR: 63.0% reduction, data not shown). Depletion of Foxp3+ cells in treated SAMP mice was appreciably lower than in other reports.16, 17 We also observed that the proportion of CD25−Foxp3+ cells increased during anti-CD25 Ab treatment in SAMP mice, compared with AKR control mice (3.92-fold vs. 1.86-fold, P<0.001, Figure 1c).
Anti-CD25 antibody (Ab) treatment depletes CD25+ cells, but not Foxp3+ cells, in SAMP mice. (a) Representative dot plots of Foxp3 and CD25 expression on mesenteric lymph node (MLN) CD4+ cells showing complete depletion of CD25+ cells in anti-CD25 Ab-treated SAMP mice (left panel). (b) The proportion of CD4+Foxp3+ cells was significantly higher in MLN cells of SAMP vs. AKR mice. The total number of CD4+Foxp3+ cells in SAMP MLNs was 3–5 times more than that observed in AKR MLNs (data not shown). Following anti-CD25 Ab treatment for 6 weeks, 28.3% of Foxp3+ cells in the MLNs of SAMP mice were eliminated, compared with 72.0% in AKR mice. (c) CD25−Foxp3+ cells significantly increased after anti-CD25 Ab treatment in MLN cells of SAMP mice, compared with AKR controls. Data are presented as the mean±s.e.m. (*P<0.05, **P<0.01, ***P<0.001). Control monoclonal antibody (mAb) SAMP, n=8; anti-CD25 Ab SAMP and control mAb AKR, n=6 each; anti-CD25 AKR, n=4.
Anti-CD25 Ab treatment increases the percentage of CD25−Foxp3+ cells in SAMP in a time-dependent fashion
We next examined the kinetics of CD25+Foxp3+ and CD25− Foxp3+ cells in the spleen of SAMP mice compared with AKR controls over the 6-week treatment period. After 1 week of Ab treatment, CD25+Foxp3+ cells from both SAMP and AKR mice decreased significantly, whereas CD25−Foxp3+ cells increased in parallel. However, the proportion of CD25−Foxp3+ cells remained increased in SAMP mice during the 6-week treatment period, but decreased to pretreatment levels in AKR mice (Figure 2a). Interestingly, CD4+ cells from anti-CD25-treated SAMP mice produced increased levels of both Th1 and Th2 cytokines compared with AKR controls, despite elevated levels of interleukin-10 (IL-10) and transforming growth factor-β (Figure 2b).
SAMP CD25−Foxp3+cells increase following anti-CD25 treatment and produce increased levels of effector cytokines. CD4+ cells from anti-CD25 antibody (Ab)-treated SAMP or AKR mice (200 μg) were isolated at 0, 1, 2, 3, and 6 weeks (n=4/time point). Mice were killed at 12 weeks of age. (a) Time course of the proportion of CD25+Foxp3+ and CD25−Foxp3+ cells in the spleen from SAMP and AKR mice. (b) Time course of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine production measured by enzyme-linked immunosorbent assay (ELISA) in triplicate from 72-h cultures of SAMP or AKR unfractionated spleen CD4+ T cells (2 × 105 cells per well) stimulated with immobilized anti-CD3 and soluble anti-CD28. Data are expressed as the mean±s.e.m. (*P<0.05, **P<0.01, ***P<0.001). IFN-γ, interferon-γ; IL, interleukin; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α.
Anti-CD25 Ab treatment increases the severity of spontaneous ileitis in SAMP mice and augments intestinal Th1 and Th2 cytokine expression
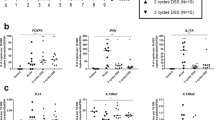
Anti-CD25 Ab treatment increased the severity of ileitis in SAMP mice (total inflammatory score=6.18±0.66 in anti-CD25 vs. 3.54±0.53 in control mAb, P<0.001, n=11 per group, Figure 3a,b). Interestingly, serum TNF-α and interferon-γ (IFN-γ) levels were also elevated in anti-CD25-treated SAMP mice compared with controls, suggesting a systemic proinflammatory effect of anti-CD25 treatment (Figure 3c). Ileal mRNA expression of both Th1 and Th2 polarized cytokines was increased in treated vs. untreated SAMP mice (Figure 3d).
Anti-CD25 antibody (Ab) treatment increases the severity of spontaneous ileitis in SAMP mice, and increases ileal expression of T helper type 1 (Th1) and T helper type 2 (Th2) cytokines. (a) Anti-CD25-treated mice developed more severe ileitis with a higher mean total inflammatory score compared with control monoclonal antibody (mAb)-treated mice; no significant colitis was detected in either group (n=11/group). (b) Representative photomicrographs of hematoxylin and eosin (H&E)-stained sections, original magnification × 10 and × 20. SAMP mice treated with isotype control Ab display minimal inflammatory changes with preservation of the villi morphology; anti-CD25-treated SAMP show increased infiltration of inflammatory cells and villous distortion. (c) Serum tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) levels measured by enzyme-linked immunosorbent assay (ELISA) were elevated in anti-CD25-treated SAMP mice compared with controls (n=6/group). (d) Total RNA was extracted from ileal tissues from anti-CD25 Ab or isotype control Ab-treated SAMP mice, and mRNA was quantified by real-time reverse-transcriptase–PCR (RT–PCR). Both Th1 and Th2 cytokines were significantly increased in anti-CD25-treated mice. Data are expressed as the mean±s.e.m. IL, interleukin. (*P<0.05, **P<0.01, ***P<0.001).
CD4+CD25+ SAMP-derived cells have normal suppressive activity in vitro
In order to test the regulatory function of SAMP Tregs in vitro, CD4+CD25− effector T cells were co-cultured with CD4+CD25+ Tregs in a standard Treg suppression assay. We observed no differences between SAMP Tregs and AKR Tregs in this assay (Supplementary Figure S2 online), suggesting that SAMP-derived MLN cells possess normal suppressive activity in vitro, similar to Tregs derived from CD patients.9
Adoptive transfer of CD4+ cells from anti-CD25 Ab-treated SAMP mice induces severe colitis in SCID recipient mice
We next assessed the colitogenic potential of CD4+ MLN cells isolated from anti-CD25 Ab-treated SAMP mice upon adoptive transfer into SCID (severe combined immunodeficient) mice. We observed that anti-CD25 Ab-treated SAMP CD4+ cells induced a more severe colitis in SCID recipients, compared with untreated SAMP CD4+ cells. Rectal prolapse was observed in 83.3% of SCID mice that received anti-CD25-treated SAMP CD4+ cells vs. 16.6% that received untreated SAMP CD4+ cells (Figure 4a); no rectal prolapse was observed in SCID mice that received anti-CD25 Ab-treated or untreated AKR CD4+ cells. SCID mice that received anti-CD25-treated SAMP CD4+ cells lost significantly more weight and had shortened colons compared with the other groups (Figure 4b,c). Total inflammatory scores in SCID mice that received anti-CD25-treated SAMP CD4+ cells were significantly higher than the other groups (anti-CD25 SAMP: 16.00±0.68 vs. control mAb SAMP: 11.50±3.28, anti-CD25 AKR: 7.00±0.58, P<0.005, control mAb AKR: 2.67±0.67, P<0.005, Figure 4d). Representative histological images of the different experimental groups are shown in Figure 4e.
Adoptive transfer of CD4+ cells from anti-CD25-treated SAMP mice induces severe colitis in SCID (severe combined immunodeficient) recipient mice. 2 × 105 mesenteric lymph node (MLN) CD4+ cells from anti-CD25-treated or isotype control monoclonal antibody (mAb)-treated SAMP mice (12 weeks, n=6/group) were transferred into adult (4–6 weeks) MHC (major histocompatibility complex)-matched SCID mice. Cells were also adoptively transferred into similarly treated AKR control mice (n=4/group). (a) Rectal prolapse occurred in 83.3% of SCID mice that received CD4+ cells from anti-CD25-treated SAMP mice vs. 16.6% that received cells from control mAb-treated SAMP mice. (b) Colon length was significantly lower in anti-CD25-treated SAMP mice. (c) Time course of body weight changes after transfer showed significant weight loss in SCID recipients transferred with cells from anti-CD25-treated SAMP compared with the other experimental groups. (d) SCID mice adoptively transferred with CD4+ MLN cells from anti-CD25-treated SAMP mice displayed higher active, chronic, and total inflammatory scores in the colon compared with mice adoptively transferred with cells from treated and untreated AKR donor mice. Data are presented as the mean±s.e.m. (*P<0.05, **P<0.01, ***P<0.001). (e) Representative sections from SCID mice adoptively transferred with CD4+ MLN cells from AKR and SAMP mice treated with either anti-CD25 or isotype control Ab. Severe colonic inflammation was observed in SCID mice receiving cells from anti-CD25 Ab SAMP donors.
CD4+CD25+ cells from SAMP mice failed to ameliorate the severity of established SCID colitis induced by CD4+ cells from anti-CD25 Ab-treated SAMP mice
To test the regulatory function(s) of CD4+CD25+ cells in vivo, we transferred this cell subpopulation from SAMP and AKR mice into colitic SCID recipients that had been previously adoptively transferred with CD4+ cells from anti-CD25 Ab-treated SAMP mice. The percentage of FoxP3+ cells in the CD25+ cell preparations was 90.8% and 88.3% in SAMP and AKR mice, respectively. Colitic SCID mice that received AKR CD4+CD25+ cells recovered as early as 2 weeks after adoptive transfer with gradual disappearance of clinical signs of intestinal inflammation and weight loss, compared with mice receiving SAMP CD4+CD25+ cells and colitic SCID controls (Figure 5a). In contrast, SCID mice injected with SAMP CD4+CD25+ cells and SCID control mice continued to lose weight and did not show evidence of clinical improvement up to 40 days following the transfer. All SCID mice that received AKR CD4+CD25+ cells survived (100%), whereas all SCID mice with no transfer died (0%), and 60% of SCID mice that received SAMP CD4+CD25+ cells survived (Figure 5b). Altogether, these results suggest that SAMP CD4+CD25+ cells may be functionally defective in vivo and have lost their regulatory properties.
SAMP CD4+CD25+ cells fail to prevent the development of adoptively transfer-induced colitis. CD4+ cells were transferred from anti-CD25-treated SAMP mice into SCID (severe combined immunodeficient) mice (6 weeks, n=10), resulting in severe colitis. After 6 weeks, 5 colitic SCID recipients per group received a second adoptive transfer of mesenteric lymph node (MLN) CD4+CD25+ cells (2 × 105) from untreated SAMP or AKR control mice (12 weeks, n=3/group). The percentage of FoxP3+ cells was 90.8% and 88.3% in SAMP and AKR mice, respectively. (a) Time course of body weight changes after transfer showed significant weight gain in SCID mice treated with AKR vs. SAMP CD4+CD25+ cells. (b) Survival analysis of different experimental groups showed 0% survival in SAMP CD4+CD25+-treated SCID mice vs. 60% in AKR CD4+CD25+-treated SCID mice. (c) Elevated levels of secreted interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) measured from 3-day cultures of SAMP (n=4 mice) or AKR (n=6 mice) MLN or spleen CD4+CD25+ T cells (2 × 105 cells per well) stimulated with immobilized anti-CD3 and soluble anti-CD28. (d) Tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-3, IL-4, IL-5, and IL-6 measured from 3-day cultures were elevated in SAMP (n=4 mice) compared with AKR (n=6 mice) MLN or spleen CD4+CD25+ T cells (2 × 105 cells per well) stimulated with immobilized anti-CD3 and soluble anti-CD28. Data are expressed as the mean±s.e.m. (*P<0.05, **P<0.01, ***P<0.001).
SAMP CD4+CD25+ cells produce regulatory cytokines, but also produce Th1 and Th2 cytokines
To characterize the immunophenotype of these cells, we measured cytokine production by CD4+CD25+ MLN or spleen cells after a 3-day culture. IL-10 production in SAMP CD4+CD25+ MLN cells was higher compared with AKR controls (Figure 5c). However, IL-3, IL-4, IL-5, IL-6, TNF-α, and IFN-γ cytokine levels were also higher in SAMP CD4+CD25+ cells compared with AKR mice (Figure 5d). These results suggest that SAMP CD4+CD25+ cells may function not only as Tregs, but also as effector T cells in vivo.
The frequency of CD4+IL-17A+ cells is decreased in SAMP mice with established ileitis following cell stimulation
To determine whether SAMP CD4+Foxp3+ Tregs express IL-17 during the development of chronic ileitis, we performed co-staining by fluorescence-activated cell sorting analysis of CD4+ MLN cells isolated from SAMP mice with established ileitis (20 weeks of age), compared with age-matched AKR control mice. We detected a very low frequency of CD4+Foxp3+IL-17A+ cells in both SAMP and AKR mice with no significant differences in both freshly isolated and stimulated MLN cells (Figure 6). However, following stimulation we detected a significant decrease in the percentage of CD4+Foxp3−IL17A+ cells in SAMP mice compared with AKR control mice (0.82±0.25% vs. 8.99±1.77%, P<0.02). As recent studies have shown that CD4+ IL17A+ cells may have regulatory functions, these results further support our hypothesis of a Treg dysregulation in SAMP mice.
The frequency of CD4+ Foxp−IL-17+ cells is decreased in SAMP mice with established ileitis. Representative CD4+ T cell-gated dot plots showing percentages of Foxp3+ and Foxp3− T cells expressing interleukin-17A (IL-17A) in mesenteric lymph nodes (MLNs) of (a) control AKR and (b) SAMP mice at 20 weeks. (c) Composite bar graph representing a significant decrease in the percentage of CD4+ Foxp3− T cells expressing IL-17A in the MLNs of SAMP vs. AKR mice; data are representative of four experiments performed separately. Data are expressed as the mean±s.e.m. (*P<0.02).
SAMP CD25−Foxp3+ cells have effector cell function in the adoptively transferred SCID model of colitis
As Foxp3 is considered the most reliable marker of Tregs, the next logical step was to investigate the specific function of Foxp3+ cells, both in vitro and in vivo. However, given that Foxp3-GFP reporter SAMP mice are not currently available, we could not isolate pure and viable populations of CD25+Foxp3+ cells from SAMP mice for adoptive transfer experiments. Therefore, we focused on CD25− cells to assess the function of the expanded SAMP CD25−Foxp3+ cell population after CD25+ cell depletion. We depleted CD25+ cells in SAMP mice using two methods: 6-week administration of anti-CD25 Ab in vivo, and isolation of CD4+CD25− cells by magnetic-activated cell sorting (MACS) in vitro. Depletion of SAMP CD4+CD25+ cells in vivo generated 10.3% Foxp3+ cells. In comparison, in vitro isolation of SAMP CD4+CD25− cells by MACS included only 1.4% Foxp3+ cells (Supplementary Figure S3A online). Next, we transferred CD4+CD25−-enriched cells (5 × 105) into SCID mice to test the function of this cell population in vivo. SCID mice that received CD4+CD25−-enriched cells either from in vitro or in vivo methods significantly lost weight compared with SCID mice that received non-depleted SAMP CD4+ cells (Supplementary Figure S3B online). There was no significant difference in body weight between SCID mice transferred with CD25−-enriched cells via either method, despite a significant difference in the number of Foxp3+ cells. Rectal prolapse was observed in 60.0% of SCID mice that received CD4+CD25−-enriched cells from either in vivo or in vitro techniques. Histological analysis revealed that SCID mice receiving CD25−-enriched cells via either method had significantly more severe colitis, compared with mice receiving SAMP MLN cells treated with control Ab (total inflammatory scores: MACS=14.00±0.7303 vs. control Ab=7.250±2.403, P<0.05; anti-CD25=13.64±1.038, P<0.05 vs. control, Supplementary Figure S3C online). These results demonstrate that CD4+CD25−Foxp3+ cells, which we observed to be increased following anti-CD25 treatment in SAMP mice, may not have a regulatory function, but rather behave as effector cells.
DISCUSSION
The results of this study reveal three novel in vivo observations regarding Treg function during CD. First, depletion of natural Tregs in SAMP mice by anti-CD25 Ab treatment increases the severity of ileitis and adoptively transferred SCID colitis. This observation supports a key role for Tregs in the development of spontaneous intestinal inflammation using an in vivo model that closely resembles CD. Second, a subsequent transfer of non-depleted CD4+CD25+ cells from AKR control mice is able to ameliorate the induced SCID colitis, whereas nondepleted CD4+CD25+ cells from SAMP mice failed to do so. These results suggest that SAMP Tregs are functionally abnormal and emphasize the potential therapeutic application of functional Tregs in the treatment of colitis. Finally, anti-CD25 Ab treatment induces a significant expansion in the number of CD25−Foxp3+ cells, specifically in SAMP mice. Although this lymphocyte subpopulation has been previously reported to have a regulatory phenotype,18 the expanded CD25−Foxp3+ cells in anti-CD25 Ab-treated SAMP mice do not possess immunoregulatory properties, but rather function as effector cells with a proinflammatory Th1/Th2 intestinal phenotype. Altogether, these results strongly suggest that Treg cells are dysfunctional in vivo and may play an important role in spontaneous CD-like intestinal inflammation.
In recent years, the potential role of Tregs in the pathogenesis of CD has been the focus of much investigation. CD patients have an expansion of CD4+Foxp3+ cells in mucosal lymphoid tissues in areas of active inflammation, such as granulomas, and these cells display ex vivo regulatory function.8 In our study, CD4+CD25+ cell depletion increased the severity of both SAMP ileitis and adoptively transferred SCID colitis. These effects were associated with elevated levels of intestinal Th1 and Th2 cytokines, which are characteristic of the chronic phase of SAMP ileitis. Genetic deletion of natural Tregs leads to autoimmunity in mice,19 and dysfunction of Tregs has been linked to human autoimmune diseases.16, 20, 21, 22 However, to our knowledge, this study represents the first demonstration that in vivo depletion of Tregs in a chronic model of spontaneous CD-like ileitis leads to a significant exacerbation of disease severity.
To investigate the regulatory function of SAMP Tregs in vivo, we performed two related adoptive transfer experiments. It is well established that transfer of Treg-depleted CD4+ cells (CD25− cells) into lymphopenic mice augments the imbalance between effector and regulatory T cells and leads to uncontrolled expansion of effector cells and colitis.23 We have previously shown that adoptive transfer of MLN CD4+ cells from germ-free SAMP mice, which contained a decreased number of Tregs, induced severe colitis in MHC (major histocompatibility complex)-matched SCID mice.15 In this study, CD4+ cells from anti-CD25 Ab-treated SAMP donors caused more severe colitis in SCID recipients than anti-CD25 Ab-treated AKR donors. However, CD4+ cells from treated SAMP mice had an increased number of Foxp3+ cells compared with treated AKR mice (9.5% vs. 2.5%), suggesting that anti-CD25 Ab treatment in SAMP mice causes a larger imbalance between CD4+ effector and regulatory T cells because of an intrinsic abnormality in Tregs. Mottet et al.6 showed that adoptive transfer of CD4+CD25+ cells prevents the development of intestinal inflammation in the T-cell transfer model of colitis. Based on our hypothesis that SAMP Tregs may be imbalanced toward an effector phenotype, we performed a second adoptive transfer experiment to further evaluate the regulatory function of SAMP CD4+CD25+ cells in vivo. In support of our hypothesis, AKR CD4+CD25+ cells were able to prevent and treat the development of colitis, whereas SAMP CD4+CD25+ cells failed to do so. To explain these findings, we propose that SAMP CD4+CD25+ cells are dysfunctional in vivo, and that a significant proportion of SAMP CD4+CD25+ cells possess activated effector T-cell functions. As evidence for this effector phenotype, SAMP CD4+CD25+ cells produce increased levels of both Th1 and Th2 cytokines compared with AKR mice, and are associated with proinflammatory effects in SAMP ileitis.14 Moreover, recent studies have shown that CD25+Foxp3+ cells may coexpress the immunoregulatory cytokine IL-17A and possess yet uncharacterized immunological functions.24 CD25+Foxp3+IL-17+ cells have been recently identified in the intestinal mucosa of patients with inflammatory bowel disease.25 In our study, we detected a very low frequency of CD4+Foxp3+IL-17A+ MLN cells in both SAMP mice with established ileitis and AKR control mice. However, following stimulation we discovered that SAMP mice produced a significantly lower number of CD4+Foxp3−IL-17A+ cells compared with AKR controls. As recent studies have shown that CD4+IL-17A+ MLN cells may possess potent immunoregulatory functions in adoptively transferred colitis,26 these results provide further evidence in support of our overall hypothesis that Treg dysfunction exists in SAMP mice. Interestingly, preliminary clinical trials using a mAb against IL-17A in patients with Crohn's disease have shown exacerbation of disease activity rather than disease amelioration, supporting an anti-inflammatory role for IL-17A in chronic intestinal inflammation (J. Wehkamp et al., unpublished data). Further characterization of this T-cell subpopulation is currently ongoing in our laboratory.
One notable finding of our study was that 6-week treatment with anti-CD25 Ab induced a significant time- and dose-dependent increase in CD25−Foxp3+ cells in SAMP mice compared with AKR controls. Recent studies have shown that IL-2 is essential for Treg homeostasis, highlighting a possible mechanism to link Treg numbers to activated T-cell responses.27, 28, 29 Indeed, IL-2-deficient mice develop intestinal inflammation at 7 weeks of age, indicating that IL-2 is more important for regulation rather than induction of inflammation in the intestinal setting.30 Interestingly, the number of Foxp3+ cells was increased in SAMP vs. AKR mice, and SAMP CD4+ cells showed increased IL-2 production, both spontaneously and following anti-CD25 treatment, and the level of IL-2 expression increased during anti-CD25 Ab treatment. These results lead us to conclude that IL-2 may be responsible for the increase in CD25−Foxp3+ cells in SAMP mice following anti-CD25 treatment.
The Foxp3+ transcription factor is considered the most reliable marker for Tregs. Ideally, the next logical step would have been to investigate the specific function of Foxp3+ cells both in vitro and in vivo. However, as Foxp3-GFP or -RFP reporter SAMP mice are not available, we could not isolate viable Foxp3+ cells for these studies. Therefore, we performed a third adoptive transfer experiment to investigate the regulatory function of SAMP CD25−Foxp3+-enriched cell populations. Our results showed that CD4+ cells from anti-CD25-treated SAMP mice, which contain 10.21% CD25−Foxp3+ cells, caused severe colitis similar to CD4+ cells from SAMP CD4+CD25− cells isolated by MACS in vitro, which contained only 1.8% CD25−Foxp3+ cells. Furthermore, CD4+ cells from anti-CD25-treated SAMP mice produced both Th1 and Th2 effector cytokines despite the marked increase in the percentage of Foxp3+ cells and elevated levels of IL-10 and transforming growth factor-β. However, the possibility exists that the 10% increase is not sufficient to overcome the effector function of the remaining CD4+CD25−Foxp3− population or that the enriched CD25−Foxp3+ population contains more effector cells. CD25−Foxp3+ cells have been previously described in mice in both lymph nodes and spleen.18 Specifically, Fontenot et al.18 demonstrated that CD4+CD25−Foxp3+ cells have regulatory function comparable to CD4+CD25+Foxp3+ Tregs in the Foxp3 knock-in mouse. Our results are consistent with studies in systemic lupus erythematosus describing increased CD25−Foxp3+ cells as dysfunctional Tregs or activated non-Tregs.31, 32, 33 Taken together, our results support our hypothesis that SAMP CD25−Foxp3+ cells may be dysfunctional and/or may acquire effector T-cell activities.
Quantitative and/or qualitative deficiencies of Tregs have been reported to contribute to the development of autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and other autoinflammatory diseases.34, 35, 36 However, in vitro functional analysis of Tregs from the peripheral blood or intestinal mucosal tissue of inflammatory bowel disease patients reveals that they maintain normal cell-contact-dependent, cytokine-independent suppressive capacity.9, 37, 38 In fact, we obtained similar results using SAMP Treg cells that display normal suppressive or regulatory activity in vitro, emphasizing the relevance of this model to the human condition. Considering the extensive evidence from mouse models and other human autoimmune diseases, it is surprising that no functional abnormalities of tissue-derived Tregs from inflammatory bowel disease patients have been reported. One explanation is that the suppressive function of Tregs in vitro may not accurately reflect their in vivo activity because of the reductionistic cell culture environment and the absence of the in vivo intestinal cytokine milieu. In fact, our study provides a comprehensive analysis of natural Treg function in a spontaneous model of CD-like ileitis and strongly supports an in vivo Treg dysfunction.
In conclusion, we have shown that in vivo depletion of Tregs by anti-CD25 Ab treatment increases the severity of intestinal inflammation in SAMP mice. Furthermore, using three adoptive transfer experiments, we have shown that Tregs in SAMP mice, including CD25−Foxp3+ cells, are dysfunctional. This study suggests that dysfunctional Tregs may play an important role in SAMP CD-like ileitis. In light of these findings, augmenting the number and/or function of Tregs in vivo may provide an effective new treatment modality for patients with autoinflammatory diseases, such as CD.
METHODS
Animals. SAMP1/YitFc (SAMP) and WT AKR mice were maintained under specific pathogen-free conditions at the Case Western Reserve University Animal Facility (Cleveland, OH). SCID (C3HSmn.C-Prkdcscid/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were maintained in accordance to approved protocols by the Institutional Animal Care and Use Committee and Association for Assessment of Laboratory Animal Care.
Antibody treatments. Anti-CD25 Ab (clone PC61) or a control rat IgG (clone HRPN, obtained from Innovative Research, Novi, MI) were dialyzed in phosphate-buffered saline, and concentrations were determined by spectrophotometry at 280 nm. Antibody was then re-suspended in phosphate-buffered saline at 1 mg ml−1 and stored at −20 °C. SAMP and AKR mice (6 weeks of age) were given intraperitoneal injections of 300 μg of anti-CD25 Ab or control Ab twice weekly for 6 weeks (total 3.6 mg).
Flow cytometry. For analysis of CD4 cells expressing CD25 and Foxp3 (1 × 106 MLN or spleen cells), cells were stained with a combination of anti-CD4-FITC, anti-CD25-APC, and anti-Foxp3-PE (Mouse Tregs staining kit, eBioscience, San Diego, CA). For analysis of Foxp3+IL-17A+ cells, either freshly isolated or stimulated (anti-CD3, 1 μg ml−1; anti-CD28, 5 μg ml−1; IL-2, 20 UI ml−1; IL-6, 20 ng ml−1; and transforming growth factor-β, 1 ng ml−1) CD4+ cells from MLNs (enriched by positive selection using CD4 microbead; Miltenyi Biotech, Cambridge, MA) were stained with anti-CD4-FITC, anti-Foxp3-APC, and anti-IL-17A-PE (eBioscience) following the manufacturer's protocol, and cells were analyzed by fluorescence-activated cell sorting (BD LSR II, San Jose, CA). Before staining, all cells were treated with anti-Fc blocking antibody. For polarized CD4 cells, cells were prestimulated with 50 ng ml−1 phorbol 12-myristate 13-acetate, 1 μg ml−1 ionomycin, and 1:1,500 diluted GolgiStop for 4 h at 37 °C and 5% CO2. The data were further analyzed using FlowJo software (Tree Star, Ashland, OR). In selected experiments, CD4+CD25+ cells were stained with either anti-CD25-APC clone PC61 (eBioscience) or clone 3C7 (BD Biosciences, Sparks, MD) with similar results.
Histological assessment. The colon and distal 10 cm of ileum from experimental mice were harvested, flushed of fecal matter, opened longitudinally, and placed in Bouin's fixative. Tissues were embedded in paraffin, cut to 3 μm, and stained with hematoxylin and eosin. Histological evaluation of ileitis was performed by a single pathologist in a blinded manner using a validated scoring system.27 Briefly, histological indices were evaluated for (i) active inflammation (infiltration with neutrophils), (ii) chronic inflammation (lymphocytes, plasma cells, and macrophages in the mucosa and submucosa), and (iii) villus distortion (flattening and/or widening of normal villus architecture). The total inflammatory score represents the sum of these three individual components. Histological evaluation of colitis was performed in hematoxylin and eosin-stained sections using a colitis scoring system calculated in a similar manner. Indices were evaluated for active inflammation, chronic inflammation, and transmural infiltration. Individual scores were summed to determine the total inflammatory score.
Real-time reverse-transcriptase–PCR. Total RNA was isolated from homogenized tissue using the RNAeasy Miniprep kit (Qiagen, Valencia, CA). Reverse transcription was performed using the GeneAmp RNA PCR kit (Applied Biosystems, Carlsbad, CA), according to the manufacturer's instructions. Amplification of IFN-γ, TNF-α, IL-4, IL-5, IL-10, IL-13, and IL-17 was performed as described previously.13, 15 Target mRNA was normalized to the 18s RNA internal control in each sample. Results were expressed as a relative ratio to the lowest control sample. All samples were assayed in duplicate.
Isolation of lymphocytes. CD4+ or CD4+CD25+ lymphocytes were purified from the MLNs using either a CD4+ or CD4+CD25+ T-cell isolation kit (MACS) according to the manufacturer's protocol (Miltenyi Biotec). Purity for CD4+ and CD4+CD25+ cells was assessed by flow cytometric analysis and was typically >95% and >90%, respectively.
Treg suppression assay. Carboxyfluorescein succinimidyl ester-labeled (0.5 μm), MACS-purified (Miltenyi Biotec) CD4+CD25− effector T cells were co-cultured with CM-Dil-labeled (1 μm) CD4+CD25+ Tregs in a ratio of 1:1 and 2:1, and activated using anti-CD3 (1 μg ml−1)/anti-CD28 (8 μg ml−1). Evaluation of cell division(s) was performed by flow cytometry based on carboxyfluorescein succinimidyl ester and CM-Dil dilutions.
Adoptive transfer. CD4+ lymphocytes from the MLNs of 12-week-old SAMP and AKR mice were positively isolated by a CD4+ T-cell isolation kit after 6 weeks of Ab treatment. Adult (6–7 weeks of age) MHC-matched SCID mice received 5 × 104 CD4+ cells per mouse by intraperitoneal injection. Mice were tested weekly for weight loss and rectal prolapse. At 6 weeks after transfer, SCID mice were killed, and terminal ilea and colons were processed for histological assessment of inflammation. Intestinal cytokine mRNA levels were measured by reverse-transcriptase–PCR; serum cytokine levels were analyzed by enzyme-linked immunosorbent assay.
CD4+ cell adoptive transfer. A total of 20 SCID mice (4–6 weeks of age) were injected intraperitoneally with 5 × 104 CD4+ cells from anti-CD25-treated SAMP mice as described above. SCID mice developed clinical signs of colitis 6 weeks after transfer. We selected 15 SCID mice that showed between 7.4 and 15.1% body weight loss and divided them into three groups at random. Next, to test the function of SAMP Tregs in vivo, we injected MLN CD4+CD25+ cells (2 × 105) isolated using a CD4+CD25+ T-cell isolation kit from adult SAMP (12 weeks of age) or AKR into adoptively transferred SCID mice that had already developed severe colitis. Body weight and clinical signs were monitored weekly. Mortality was evaluated at the end of each experiments and histologic assessment performed as described above.
Cell culture and cytokine measurement. Cultures were performed in complete medium (RPMI-1640 with 10% fetal bovine serum, 2 mm L-glutamine, 1 × 10−5 mol l−1 2-mercaptoethanol, and 1% penicillin/streptomycin). Total CD4+ cells and CD4+CD25+ cells (1 × 106 cells ml−1) from SAMP or AKR mice were cultured with 3 μg ml−1 immobilized anti-CD3 Ab and 1 μg ml−1 anti-CD28 Ab (both from BD Biosciences, Bedford, MA) for 72 h, and supernatants were harvested. Cytokines levels (TNF-α, IFN-γ, IL-2, IL-3, IL-4, IL-5, IL-6, and IL-9) were measured by Quansys Q-Plex Array Mouse IR (Quansys Bioscience, Logan, UT), following the manufacturer's instructions. TNF-α, IFN-γ, IL-10, and transforming growth factor-β protein were measured by commercially available enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Kinetics of CD25−Foxp3+ cells and cytokines from CD4+ cells following anti-CD25 treatment. Two female SAMP and AKR mice were given intraperitoneal injections of 200 μg anti-CD25 Ab weekly for 0, 1, 2, 3, or 6 weeks. Mice were killed at 12 weeks of age, and spleen CD4+ cells were analyzed by fluorescence-activated cell sorting and cell culture as described above.
Statistical analysis. Statistical analysis was performed using the two-tailed Student's t test and the Mann–Whitney U-test for nonparametric data. Quantitative data were expressed as mean±s.e.m. and differences were considered statistically significant when P<0.05.
References
Belkaid, Y. Regulatory T cells and infection: a dangerous necessity. Nat. Rev. Immunol. 7, 875–888 (2007).
Sakaguchi, S. et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27 (2006).
Himmel, M.E., Hardenberg, G., Piccirillo, C.A., Steiner, T.S. & Levings, M.K. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology 125, 145–153 (2008).
Ishimaru, N. et al. Development of inflammatory bowel disease in Long-Evans Cinnamon rats based on CD4+CD25+Foxp3+ regulatory T cell dysfunction. J. Immunol. 180, 6997–7008 (2008).
Izcue, A., Coombes, J.L. & Powrie, F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27, 313–338 (2009).
Mottet, C., Uhlig, H.H. & Powrie, F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170, 3939–3943 (2003).
Read, S. & Powrie, F. Induction of inflammatory bowel disease in immunodeficient mice by depletion of regulatory T cells. Curr. Protoc. Immunol. Chapter 15: Unit 15.13 (2001).
Saruta, M. et al. Characterization of FOXP3+CD4+ regulatory T cells in Crohn's disease. Clin. Immunol. 125, 281–290 (2007).
Kelsen, J. et al. FoxP3(+)CD4(+)CD25(+) T cells with regulatory properties can be cultured from colonic mucosa of patients with Crohn's disease. Clin. Exp. Immunol. 141, 549–557 (2005).
Rivera-Nieves, J. et al. Emergence of perianal fistulizing disease in the SAMP1/YitFc mouse, a spontaneous model of chronic ileitis. Gastroenterology 124, 972–982 (2003).
Sugawara, K. et al. Linkage to peroxisome proliferator-activated receptor-gamma in SAMP1/YitFc mice and in human Crohn's disease. Gastroenterology 128, 351–360 (2005).
Marini, M. et al. TNF-alpha neutralization ameliorates the severity of murine Crohn's-like ileitis by abrogation of intestinal epithelial cell apoptosis. Proc. Natl. Acad. Sci. USA 100, 8366–8371 (2003).
Kosiewicz, M.M. et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease. J. Clin. Invest. 107, 695–702 (2001).
Bamias, G. et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology 128, 654–666 (2005).
Bamias, G. et al. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J. Immunol. 178, 1809–1818 (2007).
Sakaguchi, S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Tenorio, E.P., Olguin, J.E., Fernandez, J., Vieyra, P. & Saavedra, R. Reduction of Foxp3+ cells by depletion with the PC61 mAb induces mortality in resistant BALB/c mice infected with Toxoplasma gondii. J. Biomed. Biotechnol. 2010, 786078 (2009).
Fontenot, J.D. et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22, 329–341 (2005).
Brunkow, M.E. et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27, 68–73 (2001).
Liu, M.F., Wang, C.R., Fung, L.L. & Wu, C.R. Decreased CD4+CD25+ T cells in peripheral blood of patients with systemic lupus erythematosus. Scand J. Immunol. 59, 198–202 (2004).
Suri-Payer, E., Amar, A.Z., Thornton, A.M. & Shevach, E.M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 160, 1212–1218 (1998).
Viglietta, V., Baecher-Allan, C., Weiner, H.L. & Hafler, D.A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 199, 971–979 (2004).
Ostanin, D.V. et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G135–G146 (2009).
Ayyoub, M. et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc. Natl. Acad. Sci. USA 106, 8635–8640 (2009).
Hovhannisyan, Z., Treatman, J., Littman, D.R. & Mayer, L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology 140, 957–965 (2011).
O'Connor, W. Jr . et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10, 603–609 (2009).
Dooms, H. & Abbas, A.K. Revisiting the role of IL-2 in autoimmunity. Eur. J. Immunol. 40, 1538–1540 (2010).
Fontenot, J.D., Rasmussen, J.P., Gavin, M.A. & Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Malek, T.R. The biology of interleukin-2. Annu. Rev. Immunol. 26, 453–479 (2008).
Autenrieth, I.B., Bucheler, N., Bohn, E., Heinze, G. & Horak, I. Cytokine mRNA expression in intestinal tissue of interleukin-2 deficient mice with bowel inflammation. Gut 41, 793–800 (1997).
Bonelli, M. et al. Phenotypic and functional analysis of CD4+ CD25− Foxp3+ T cells in patients with systemic lupus erythematosus. J. Immunol. 182, 1689–1695 (2009).
Horwitz, D.A. Identity of mysterious CD4+CD25-Foxp3+ cells in SLE. Arthritis Res. Ther. 12, 101 (2010).
Yang, H.X. et al. Are CD4+CD25-Foxp3+ cells in untreated new-onset lupus patients regulatory T cells? Arthritis Res. Ther. 11, R153 (2009).
Coombes, J.L., Robinson, N.J., Maloy, K.J., Uhlig, H.H. & Powrie, F. Regulatory T cells and intestinal homeostasis. Immunol. Rev. 204, 184–194 (2005).
Ehrenstein, M.R. et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J. Exp. Med. 200, 277–285 (2004).
Venken, K. et al. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 123, 79–89 (2008).
Bour-Jordan, H. & Bluestone, J.A. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol. Rev. 229, 41–66 (2009).
La Cava, A. Tregs are regulated by cytokines: implications for autoimmunity. Autoimmun. Rev. 8, 83–87 (2008).
Acknowledgements
We thank Mitchell Guanzon and Dennis Gruszka for technical support. We also thank Rehka Garg for assistance with the in vitro suppression assay. This study was funded by the National Institutes of Health, DK 055812, DK 042191, and DK 091222 to F.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared no conflict of interest.
Additional information
SUPPLEMENTARY MATERIAL is linked to the online version of the paper
Rights and permissions
About this article
Cite this article
Ishikawa, D., Okazawa, A., Corridoni, D. et al. Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn's disease. Mucosal Immunol 6, 267–275 (2013). https://doi.org/10.1038/mi.2012.67
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mi.2012.67
This article is cited by
-
Harnessing murine models of Crohn's disease ileitis to advance concepts of pathophysiology and treatment
Mucosal Immunology (2022)
-
Aging impairs arterial compliance via Klotho-mediated downregulation of B-cell population and IgG levels
Cellular and Molecular Life Sciences (2022)
-
Alternatively Activated Macrophages Are the Primary Retinoic Acid-Producing Cells in Human Decidua
Reproductive Sciences (2020)
-
Inflammatory bowel disease: between genetics and microbiota
Molecular Biology Reports (2020)
-
Loss of estrogen-mediated immunoprotection underlies female gender bias in experimental Crohn's-like ileitis
Mucosal Immunology (2014)