Abstract
The single-arm, phase 2 ENESTfreedom trial assessed the potential for treatment-free remission (TFR; i.e., the ability to maintain a molecular response after stopping therapy) following frontline nilotinib treatment. Patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase with MR4.5 (BCR-ABL1⩽0.0032% on the International Scale (BCR-ABL1IS)) and ⩾2 years of frontline nilotinib therapy were enrolled. Patients with sustained deep molecular response during the 1-year nilotinib consolidation phase were eligible to stop treatment and enter the TFR phase. Patients with loss of major molecular response (MMR; BCR-ABL1IS⩽0.1%) during the TFR phase reinitiated nilotinib. In total, 215 patients entered the consolidation phase, of whom 190 entered the TFR phase. The median duration of nilotinib before stopping treatment was 43.5 months. At 48 weeks after stopping nilotinib, 98 patients (51.6%; 95% confidence interval, 44.2–58.9%) remained in MMR or better (primary end point). Of the 86 patients who restarted nilotinib in the treatment reinitiation phase after loss of MMR, 98.8% and 88.4%, respectively, regained MMR and MR4.5 by the data cutoff date. Consistent with prior reports of imatinib-treated patients, musculoskeletal pain-related events were reported in 24.7% of patients in the TFR phase (consolidation phase, 16.3%).
Similar content being viewed by others
Introduction
Treatment-free remission (TFR; that is, sustained major molecular response (MMR; BCR-ABL1⩽0.1% on the International Scale (BCR-ABL1IS)) or deep molecular response (DMR) after stopping tyrosine kinase inhibitor (TKI) therapy) is an emerging treatment goal for patients with chronic myeloid leukemia in chronic phase (CML-CP). Current recommendations of the European LeukemiaNet call for indefinite treatment with TKIs in responding patients,1 whereas the 2017 National Comprehensive Cancer Network guidelines for CML note that discontinuation of TKI therapy is feasible in selected patients in the context of careful monitoring.2 Patients may be motivated to stop therapy for reasons including relief from treatment side effects, the ability to safely attempt pregnancy or the desire for other potential improvements in health-related quality of life.3, 4, 5 The potential for patients who achieve DMR to remain in remission after stopping TKI therapy was first demonstrated in the Stop Imatinib 1 trial, in which ≈40% of patients with sustained DMR (with undetectable BCR-ABL1 for ⩾2 years) on long-term imatinib maintained this response (no confirmed loss of undetectable BCR-ABL1) 1 year after stopping treatment.6
Since the Stop Imatinib 1 trial, several additional trials have investigated TKI discontinuation in patients with sustained DMR, the majority of which involved patients treated with long-term imatinib therapy.4, 7, 8, 9, 10, 11, 12, 13 However, the European Stop Tyrosine Kinase Inhibitor (EURO-SKI) study—the largest TFR trial to date10—includes patients who achieved sustained DMR (specifically, MR4 (BCR-ABL1IS⩽0.01%) for ⩾1 year) on imatinib, nilotinib or dasatinib. Other ongoing trials are specifically investigating TFR following treatment with second-generation TKIs, including the STOP 2G-TKI study of patients with undetectable BCR-ABL1 for ⩾2 years on frontline or second-line nilotinib or dasatinib,14 the Dasatinib Discontinuation study of patients on second- or later-line dasatinib with DMR (BCR-ABL1<0.0069%) for ⩾1 year12 and a series of four Evaluating Nilotinib Efficacy and Safety in Clinical Trials (ENEST) studies assessing TFR in nilotinib-treated patients: ENESTfreedom in patients administered frontline nilotinib and ENESTop, ENESTgoal and ENESTpath in distinct populations of patients administered second-line nilotinib.15, 16, 17 The ongoing single-arm, phase 2 ENESTfreedom trial is the first study to assess specifically the potential for patients with sustained DMR during frontline nilotinib to stop treatment. Here we present the first results and the results of the primary end point analysis from ENESTfreedom with a minimum follow-up of 48 weeks after stopping nilotinib.
Materials and methods
Patients, study design and treatment
Patients (aged ⩾18 years) with Philadelphia chromosome-positive CML-CP who had ⩾2 years of frontline treatment with nilotinib and achieved MR4.5 (BCR-ABL1IS⩽0.0032%; determined by a designated laboratory (MolecularMD, Portland, OR, USA) in a sample with ⩾32 000 ABL1 copies) were eligible. Evidence of typical BCR-ABL1 transcripts (that is, b3a2 (e14a2) and/or b2a2 (e13a2)) at the time of diagnosis was required. Patients who had previously received other BCR-ABL1 TKIs (cumulative duration >4 weeks) or interferon-alfa (any duration) or were unable to tolerate a minimum dose of nilotinib 400 mg once daily were not eligible.
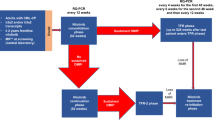
Upon enrollment all patients entered a 1-year consolidation phase during which they continued nilotinib treatment (Figure 1). Molecular responses were assessed every 12 weeks during the year of consolidation treatment by real-time quantitative polymerase chain reaction (RQ-PCR); sustained DMR was defined as MR4.5 in the last assessment, no assessment worse than MR4 and⩽2 assessments between MR4 and MR4.5. Patients with sustained DMR throughout the consolidation phase were eligible to enter the TFR phase and stop nilotinib treatment. Patients with loss of MMR during the TFR phase were required to reinitiate nilotinib 300 mg two times daily (or their previously tolerated dose) in the treatment reinitiation phase. Patients who did not sustain DMR during the consolidation phase remained on nilotinib treatment (continuation phase; Supplementary Appendix).
Study end points and assessments
The primary efficacy end point was the proportion of patients who were in MMR without reinitiation of treatment at week 48 of the TFR phase. Secondary end points included the proportion of patients who were in MR4.5 and off treatment at 48 weeks from the start of the TFR phase, treatment-free survival (TFS) over time (defined as the time from the start of TFR until the earliest occurrence of any of the following: loss of MMR, reinitiation of nilotinib for any reason, progression to accelerated phase (AP)/blast crisis (BC) or death because of any cause), reachievement of MMR and MR4.5 after nilotinib reinitiation and safety.
Molecular responses were measured as ratios of BCR-ABL1 to ABL1 expressed on the IS, determined from RQ-PCR assessments of peripheral blood conducted in a central laboratory. During the TFR phase, molecular responses were evaluated every 4 weeks for the first 48 weeks, every 6 weeks for the next 48 weeks and every 12 weeks thereafter. Patients with loss of MR4 during the TFR phase were monitored every 2 weeks until they regained MR4, remained in MMR without regaining MR4 for 8 weeks (at which point the patient received monthly monitoring) or lost MMR and entered the treatment reinitiation phase. During the treatment reinitiation phase, molecular responses were evaluated every 4 weeks for the first 24 weeks and every 12 weeks thereafter (or as clinically indicated for patients who had not regained MMR). When sufficient sample was available, mutational analysis by Sanger sequencing was performed for patients with loss of MMR, or as clinically indicated.
Adverse events (AEs) were assessed according to the Common Terminology Criteria for Adverse Events version 4.03. Evaluation of AEs and laboratory abnormalities were conducted on an ongoing basis on study and for up to 30 days after the last dose of study treatment or last day of TFR.
The impact of TFR on patients’ quality of life was assessed as an exploratory end point. At protocol-defined time points throughout the study, patient-reported outcomes were collected using the MD Anderson Symptom Inventory for CML and EuroQol visual analog scale to obtain overall quality-of-life scores, as well as the EuroQol EQ-5D-5L questionnaire, in which patients reported the presence or absence and severity of problems experienced related to mobility, self-care, usual activities, pain/discomfort and anxiety/depression.18, 19
Statistical analyses
The planned sample size was based on Fleming’s single-stage design and the minimum number of patients required to reject the null hypothesis for the primary end point (⩽50% TFR rate at 48 weeks). Assuming that ≈30% of enrolled patients would not qualify for the TFR phase, a minimum enrollment of 175 patients was required to achieve 90% power to reject the null hypothesis with a one-sided α-level of 2.5% if the true TFR rate at 48 weeks was ⩾65%. With an actual enrollment of 215 patients, the power increased to 94% with all the other assumptions being the same.
Demographics, baseline characteristics, efficacy and safety results are reported for patients who entered the TFR phase (that is, the TFR population). The primary end point was presented as a percentage with an exact 95% Clopper–Pearson confidence interval (CI). TFS was estimated using the Kaplan–Meier method; survival time for patients without an event was censored at the date of the last assessment. Rates of MMR and MR4.5 regained in the treatment reinitiation phase were reported as cumulative incidences. An analysis of baseline factors as potential predictors of TFR maintenance was conducted using a multivariate logistic regression analysis. The MMR rate at 48 weeks and TFS were also analyzed in patients who stayed in the TFR phase for ⩾24 weeks. The frequencies of AEs, laboratory abnormalities and predefined groupings of AE types of special interest were summarized for the TFR population by study phase (the consolidation phase and the first 48 weeks of the TFR phase).
The data presented herein are based on a cutoff date of 30 November 2015, at which time all patients who entered the TFR phase had completed ⩾48 weeks of TFR, entered the treatment reinitiation phase or discontinued the study.
Ethics
This study was designed and conducted in accordance with the ICH Harmonized Tripartite Guidelines for Good Clinical Practice and the ethical principles of the Declaration of Helsinki. All patients provided written informed consent before any study procedures and in accordance with local laws/regulations. The study protocol and amendments were reviewed by an independent ethics committee or institutional review board for each study center.
Results
Patients
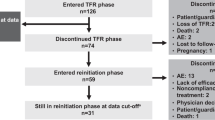
A total of 231 patients were screened for eligibility between 4 March 2013 and 22 November 2013; 215 of these patients enrolled and entered the consolidation phase. Two hundred and three patients completed the consolidation phase, including 190 (88.4%) who entered the TFR phase and 13 who entered the continuation phase (due to no sustained DMR during the consolidation phase). Twelve patients discontinued from the study during the consolidation phase (Figure 2), including two patients who discontinued with sustained DMR (due to patient decision). In the TFR population (n=190), the median time from first achievement of MR4.5 to study entry was 18.3 months, and the median duration of nilotinib before TFR was 43.5 months (Table 1).
TFR phase
At week 48 of the TFR phase, 98 patients (51.6%; 95% CI, 44.2–58.9 (null hypothesis could not be rejected because of the lower limit of the 95% CI being ⩽50%)) remained in MMR without treatment reinitiation; of these 98 patients, 5 had confirmed loss of MR4 by 48 weeks (1 of these 5 patients went on to lose MMR after 48 weeks but before the data cutoff date), 3 had loss of MR4.5 without confirmed loss of MR4 and 90 (47.4% of all 190 patients who stopped treatment; 95% CI, 40.1–54.7) had MR4.5 or better at 48 weeks. Ninety-nine of 190 patients did not have a TFS event by the data cutoff date (96 remained in TFR and 3 discontinued from the study (due to patient decision) while in TFR without experiencing a TFS event and were censored in the analysis), whereas 91 patients (47.9%) had TFS events (86 entered the reinitiation phase, 1 died, 3 discontinued TFR without entering the reinitiation phase (1 due to physician decision and 2 due to loss of MMR) and 1 lost MMR at TFR week 48 but remained in the TFR phase at the data cutoff). Most TFS events (70 of 91) occurred within the first 24 weeks (Figure 3); of 120 patients remaining in TFR at 24 weeks, 98 (81.7%) had MMR at 48 weeks (Supplementary Figure S1).
Of the 89 patients with loss of MMR while off nilotinib, 72 had an evaluable mutational assessment, and 1 patient (0.5%) had a detectable BCR-ABL1 mutation (F359V). Whether this mutation was pre-existing could not be determined because of the low BCR-ABL1 copy number in all prior samples collected during the study; however, the mutation was not detected in samples collected at initiation of nilotinib therapy and after 2 years on therapy. This patient regained MMR with nilotinib retreatment, subsequently lost MMR on nilotinib, and discontinued from the study owing to the lack of efficacy.
Median age, sex distribution and median durations of nilotinib and DMR were similar among patients with or without TFR at 48 weeks (Supplementary Tables S1 and S2). In a multivariate logistic regression analysis for TFR at 48 weeks with baseline factors as explanatory variables in the model, no strong predictors were formally identified.
Treatment reinitiation phase
Of the 86 patients who entered the treatment reinitiation phase (median duration of nilotinib retreatment, 39.6 weeks (range, 5.0–69.7 weeks)), 85 and 76 regained MMR and MR4.5, respectively, by the data cutoff date (Figure 4). The patient who did not regain MMR after restarting nilotinib withdrew consent and discontinued from the study after 7.1 weeks of nilotinib retreatment. Of the nine patients who regained MMR but not MR4.5 by the data cutoff date, four remained in the treatment reinitiation phase and five discontinued from the study due to AEs (n=2), lack of efficacy (n=1 (patient with F359V mutation)), physician decision (n=1) or patient decision (n=1).
Quality of life
Mean baseline quality-of-life scores were high (mean (s.d.) score at week 48 of the consolidation phase, which was considered the baseline for the TFR phase: MD Anderson Symptom Inventory for CML severity, 1.4 (1.41); MD Anderson Symptom Inventory for CML interference, 1.7 (2.31); EuroQol visual analog scale, 80.5 (15.61)). Minimal changes in quality-of-life scores after stopping or reinitiating treatment were detected among evaluable patients. The proportions of patients reporting problems in each dimension of the EQ-5D-5L tended to be similar across study phases, although a slightly higher proportion of patients reported problems (of any severity) with pain/discomfort after stopping treatment (49.0% of evaluable patients reported problems at week 48 of the consolidation phase compared with 58.1% at week 12 of the TFR phase).
Safety
No patients progressed to AP/BC. Five deaths were reported by the data cutoff date: two deaths occurred during the consolidation phase (one cardiac arrest and one suicide), one occurred during the TFR phase (unknown cause) and two occurred during the treatment reinitiation phase (one acute myocardial infarction and one due to an unknown cause).
Among patients who entered the TFR phase, AEs were reported in 83.2% during the 1-year consolidation phase (grade 3/4 in 13.7%) and in 65.8% during the first 48 weeks of the TFR phase (grade 3/4 in 11.1%). The most common AEs during the consolidation and TFR phases were nasopharyngitis (11.1%) and arthralgia (12.1%), respectively (Table 2). Elevations in glucose, alanine aminotransferase, aspartate aminotransferase, bilirubin and lipase were less common in the TFR phase than in the consolidation phase. Most patients with laboratory abnormalities during the TFR phase had experienced the same abnormalities during the consolidation phase. No notable differences in hematologic abnormalities were reported during the consolidation phase compared with the TFR phase (Table 2). Cardiovascular events (CVEs) were reported in four patients (2.1%) during the consolidation phase (ischemic heart disease (n=2), ischemic cerebrovascular event and peripheral artery disease (n=1 each)) and in five patients (2.6%) during the TFR phase (ischemic cerebrovascular events and peripheral artery disease (n=2 each) and arteriosclerosis (n=1), all of which were first reported during the first 24 weeks of the TFR phase; Table 3). One patient had CVEs reported during both the consolidation and TFR phases. No patient in the TFR population discontinued from the study because of a CVE.
Forty-seven patients (24.7%) had AEs in a predefined musculoskeletal pain grouping (consisting of AEs reported as musculoskeletal pain, myalgia, pain in extremity, arthralgia, bone pain and spinal pain) during the first 48 weeks of the TFR phase compared with 31 patients (16.3%) during the 1-year consolidation phase. Of the 47 patients with musculoskeletal pain-related events in the TFR phase, 45 had grade 1/2 events and 2 had grade 3 events (none led to study discontinuation; including 1 patient with grade 3 arthralgia and 1 patient with 2 episodes of grade 3 bone pain); musculoskeletal pain-related events were more frequent in female (33.0%) than in male patients (18.8%) during the TFR phase (Table 4). Thirty-two of the 47 patients had no history of musculoskeletal pain in the consolidation phase or before enrollment. Most AEs in the musculoskeletal pain grouping (n=39) were reported within 24 weeks of stopping nilotinib. The Kaplan–Meier-estimated median duration of these AEs was 24.0 weeks (95% CI, 10.1 weeks—not estimable).
Discussion
In ENESTfreedom, 51.6% (95% CI, 44.2–58.9) of patients remained in remission at 48 weeks after stopping nilotinib, consistent with results from other TFR studies following long-term TKI treatment.4, 7, 8, 9, 10, 11, 12, 13 As the prespecified statistical null hypothesis (TFR rate⩽50%) could not be rejected because of the lower limit of the 95% CI being ⩽50%, the primary end point failed statistically. This is a result of selecting too high of a threshold (50%) for the TFR rate in the null hypothesis, which was due to an underestimation of the impact of the TKI treatment duration on TFR rate at the time the protocol was developed. Nonetheless, the observed TFR rate of 51.6% is a clinically important outcome, particularly when considering the relatively short duration of prior nilotinib therapy (3.6 years) among patients in the study and the association between duration of TKI therapy and TFR probability in prior studies.4, 10 Comparison of results across TFR trials is limited by study design variations (for example, depth/duration of DMR required before stopping treatment, minimal duration of TKI therapy before stopping treatment and definition of loss of remission); however, the ability to remain in remission after stopping TKI therapy has been consistently demonstrated in patients with sustained DMR on long-term TKI treatment.4, 6, 7, 8, 9, 10, 11, 12, 13 In EURO-SKI, among 772 patients eligible to stop imatinib, nilotinib or dasatinib after a median treatment duration of 91 months and with MR4 sustained for ⩾1 year, molecular relapse-free survival at 12 months was 56% (95% CI, 52–59).10
Stopping frontline nilotinib was safe in ENESTfreedom: no new safety signals were identified during treatment, nearly all patients who reinitiated nilotinib after loss of MMR regained MMR (98.8%) and MR4.5 (88.4%) rapidly, and no patient progressed to AP/BC. The majority of patients with loss of MMR lost the response within 6 months of stopping nilotinib, highlighting the importance of frequent monitoring of patients who stop TKI therapy to ensure timely retreatment. One patient with loss of MMR during the TFR phase was found to have a detectable mutation; however, without serial testing of prior samples, the significance of this finding is unclear because the mutation may have been present before the patient started the TFR phase. Additionally, because low-level mutations can be undetectable using Sanger sequencing,20 it is possible that other patients may have had kinase domain mutations below the limit of detection. However, the emergence of BCR-ABL1 mutations may be less likely during the TFR phase because of the absence of TKI-induced selective pressure for mutants. Kinase domain mutations have not been reported in other TFR studies.4, 6, 7, 8, 9, 10, 11, 12, 13
Although long-term safety/tolerability considerations are motivators for stopping treatment in some patients,3, 4, 5 the rates of some AEs (for example, hematologic abnormalities and CVEs) were similar in the first 48 weeks of the TFR phase compared with the consolidation phase. This may be due to the fact that this study enrolled patients who had already received nilotinib for multiple years and had established tolerance of nilotinib. Further follow-up is required to determine whether the risk of CVEs and other AEs decreases over time after stopping treatment. In addition, the frequency of low-grade musculoskeletal pain-related events increased during the first year of the TFR phase, consistent with prior reports in patients who stopped imatinib therapy.4, 21, 22, 23, 24 In subanalyses of (mostly imatinib-treated) patients from EURO-SKI, ~1/3 experienced musculoskeletal pain after stopping treatment.21, 22, 24 Similar events were retrospectively described in 30% (n=27/90) of patients in the Korean Imatinib Discontinuation study.4 Similar to the findings in ENESTfreedom, the majority of these events were reported within 6 months of stopping therapy.4 Many of these events were self-limiting, resolving within 12 months, although some events continued beyond 1 year.4, 21
No impact of stopping treatment on patients’ overall quality of life was detected. This may be due to patients having a relatively high quality of life before treatment discontinuation; it may also result from the use of questionnaires that were not optimal for assessment of patients doing well on treatment.
Recent reviews by Saussele et al.25 and Hughes and Ross26 have discussed potential criteria for identifying patients who may be candidates for TFR and recommendations for monitoring of patients during TFR based on available data from clinical trials to date. Both reviews highlight the importance of sustained DMR before stopping treatment and of frequent patient monitoring, including quantification of typical BCR-ABL1 transcript levels by a well-validated assay run in an IS-standardized center following established guidelines for measuring DMR.27 Identification of additional prognostic criteria for TFR remains under investigation.25, 26 Although no strong prognostic factors were detected in this study, the analysis may have been limited by the size and relative homogeneity of the population (for example, most patients had a similar prior treatment duration).
Frontline nilotinib therapy is known to result in rapid and high rates of DMR28, 29 —a key prerequisite for TFR.4, 6, 7, 8, 9, 10, 11, 12, 13 In ENEST1st, 55.2% (n=581/1052) and 38.6% (n=406/1052) of patients achieved MR4 and MR4.5, respectively, by 2 years with frontline nilotinib.29 In ENESTnd, more than half of patients treated with frontline nilotinib 300 mg two times daily achieved MR4.5 by 5 years (n=151/282; 54%), compared with 31% of patients treated with frontline imatinib (n=89/283).28 Furthermore, with 6 years of follow-up in ENESTnd, 38% (n=107/282) of patients in the nilotinib 300-mg twice-daily arm vs 22% (n=61/283) in the imatinib arm met the treatment duration and sustained DMR requirements defining eligibility to attempt TFR in ENESTfreedom.30 These data suggest that frontline nilotinib may increase the number of patients who become eligible to discontinue treatment.
When selecting a TKI for frontline therapy, several factors must be considered: in addition to the efficacy and safety profiles of each available treatment option, treatment cost can be an important consideration, particularly with the introduction of generic imatinib; now, the increased potential for TFR eligibility with nilotinib (and the potential cost savings through treatment discontinuation31, 32, 33) may be additional factors to consider for some patients when selecting a frontline TKI. These long-term considerations are increasingly important as patients with CML now have a life expectancy comparable to that of the general population.34
As the first study to specifically investigate TFR following frontline nilotinib, ENESTfreedom can provide important new information on this emerging treatment goal for patients with CML-CP. Here, the first results from ENESTfreedom demonstrate that a clinically significant percentage of patients (51.6%) with sustained DMR on frontline nilotinib therapy and a median treatment duration of 43.5 months were able to remain in MMR for ⩾48 weeks after stopping nilotinib. The results from ENESTfreedom, together with the results from ENESTnd showing higher rates of DMR and sustained DMR with nilotinib vs imatinib,28, 30 suggest that more patients may become eligible to stop treatment and sustain remission following frontline nilotinib therapy than following imatinib therapy. Additional follow-up and analyses of TFR data in ENESTfreedom and other TFR studies will be needed to further evaluate the patient, disease and treatment characteristics before stopping treatment that may be associated with maintaining TFR, as well as the long-term durability of TFR.
References
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood 2013; 122: 872–884.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Chronic Myeloid Leukemia, v1.2017. Available at: https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf (last accessed 6 December 2016).
Boquimpani CM, Szczudlo T, Mendelson E, Benjamin K, Masszi T . Attitudes and perceptions of patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) toward treatment-free remission (TFR). Blood 2014; 124, (abstract 4547).
Lee SE, Choi SY, Song HY, Kim SH, Choi MY, Park JS et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica 2016; 101: 717–723.
Ross D, Villemagne-Sanchez L, Hall K, Gough K, Kashima Y, O'Callaghan C et al. Factors that influence patient willingness to attempt treatment-free remission in chronic myeloid leukaemia. Haematologica 2016; 101: 238.
Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010; 11: 1029–1035.
Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol 2014; 32: 424–430.
Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER Study. Blood 2013; 122: 515–522.
Mori S, Le Coutre P, Abruzzese E, Martino B, Pungolino E, Elena C et al. Late relapses, up to 45 months after imatinib discontinuation: results from the ISAV study. Haematologica 2016; 101: 62–63.
Richter J, Mahon FX, Guilhot J, Hjorth-Hansen H, Almeida A, Janssen JJ et al. Stopping tyrosine kinase inhibitors in a very large cohort of European chronic myeloid leukemia patients: results of the EURO-SKI trial. Haematologica 2016; 101: 22–23.
Rea D, Nicolini FE, Tulliez M, Rousselot P, Guilhot F, Gardembas M et al. Dasatinib or nilotinib discontinuation in chronic phase (CP)-chronic myeloid leukemia (CML) patients (pts) with durably undetectable BCR-ABL transcripts: interim analysis of the STOP 2G-TKI study with a minimum follow-up of 12 months—on behalf of the French CML Group Filmc. Blood 2014; 124: (abstract 811).
Imagawa J, Tanaka H, Okada M, Nakamae H, Hino M, Murai K et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol 2015; 2: e528–e535.
Takahashi N, Nishiwaki K, Nakaseko C, Wakita H, Sano K, Ohwada C et al. Successful treatment free remission in CML after 2 year consolidation with nilotinib of an MR4.5 response level achieved originally with imatinib treatment: first report from STAT2 trial in Japan. Haematologica 2016; 101: 61.
Rea D, Rousselot P, Guilhot F, Tulliez M, Nicolini FE, Guerci-Bresler A et al. Discontinuation of second generation (2G) tyrosine kinase inhibitors (TKI) in chronic phase (CP)-chronic myeloid leukemia (CML) patients with stable undetectable BCR-ABL transcripts. Blood 2012; 120: (abstract 916).
Hughes TP, Boquimpani C, Takahashi N, Benyamini N, Clementino NCD, Shuvaev V et al. Results from ENESTop: treatment-free remission following switch to nilotinib in patients with chronic myeloid leukemia in chronic phase. Haematologica 2016; 101: 65.
Ritchie EK, Catchatourian R, Klisovic RB, Deininger MW, Erba HP, Radich JP et al. Rapid achievement of MR4.5 after switching from imatinib (IM) to nilotinib (NIL) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP): preliminary results from ENESTgoal. Blood 2015; 126: (abstract 4050).
Rea D, Rosti G, Cross NCP, Hellman A, Niederwiester D, Pungolino E et al. Enestpath: a phase III study to assess the effect of nilotinib treatment duration on treatment-free remission (TFR) in chronic phase-chronic myeloid leukemia (CP-CML) patients (pts) previously treated with imatinib: interim analysis from the first year of induction phase. Blood 2015; 126: (abstract 4040).
Williams LA, Garcia Gonzalez AG, Ault P, Mendoza TR, Sailors ML, Williams JL et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood 2013; 122: 641–647.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–1736.
Jones D, Kamel-Reid S, Bahler D, Dong H, Elenitoba-Johnson K, Press R et al. Laboratory practice guidelines for detecting and reporting BCR-ABL drug resistance mutations in chronic myelogenous leukemia and acute lymphoblastic leukemia: a report of the Association for Molecular Pathology. J Mol Diagn 2009; 11: 4–11.
Richter J, Söderlund S, Lübking A, Dreimane A, Lotfi K, Markevärn B et al. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J Clin Oncol 2014; 32: 2821–2823.
Mahon FX, Richter J, Guilhot J, Muller MC, Dietz C, Porkka K et al. Interim analysis of a pan European stop tyrosine kinase inhibitor trial in chronic myeloid leukemia: the EURO-SKI study. Blood 2014; 124: (abstract 151).
Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B et al. Reply to J. Richter et al. J Clin Oncol 2014; 32: 2823–2825.
Berger MG, Perieira B, Oris C, Saugues S, Cony-Makhoul P, Gardembas M et al. Osteoarticular pain after discontinuation of tyrosine kinase inhibitors (TKI): a French cohort. Blood 2015; 126: (abstract 137).
Saussele S, Richter J, Hochhaus A, Mahon FX . The concept of treatment-free remission in chronic myeloid leukemia. Leukemia 2016; 30: 1638–1647.
Hughes TP, Ross DM . Moving treatment-free remission into mainstream clinical practice in CML. Blood 2016; 128: 17–23.
Cross NCP, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E et al. Laboratory recommendations for scoring deep molecular response following treatment for chronic myeloid leukemia. Leukemia 2015; 29: 999–1003.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Kim DW, Issaragrisil S et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia 2016; 30: 1044–1054.
Hochhaus A, Rosti G, Cross NCP, Steegmann JL, le Coutre P, Ossenkoppele G et al. Frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the European ENEST1st study. Leukemia 2016; 30: 57–64.
Hochhaus A, Saglio G, Hughes TP, Larson RA, Taningco L, Deng W et al. Impact of treatment with frontline nilotinib (NIL) vs imatinib (IM) on sustained deep molecular response (MR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP). Blood 2015; 126: (abstract 2781).
Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini FE et al. Long term follow-up after imatinib cessation for patients indeep molecular response: the update results of the STIM1 study. Blood 2013; 122: (abstract 255).
Mahon FX, Nicolini FE, Noël M, Escoffre M, Charbonnier A, Rea D et al. Preliminary report of the STIM2 study: a multicenter stop imatinib trial for chronic phase chronic myeloid leukemia de novo patients on imatinib. Blood 2013; 122: (abstract 654).
Mahon F, Richter J, Guilhot J, Hjorth-Hansen H, Almeida A, Janssen JWM et al. Cessation of tyrosine kinase inhibitors treatment in chronic myeloid leukemia patients with deep molecular response: results of the Euro-Ski trial. Blood 2016; 128: (abstract 787).
Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM . Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol 2016; 34: 2851–2857.
Acknowledgements
We thank all 114 study sites and participating countries (including Argentina, Austria, Belgium, Bulgaria, Colombia, Denmark, France, Germany, Greece, Hungary, Ireland, Italy, Japan, the Netherlands, Poland, Spain, Sweden, the United Kingdom and the United States) as well as MolecularMD for conducting the laboratory analyses, the Novartis team and all enrolled patients for their participation. We also thank Karen Kaluza, PhD, and Jonathan Morgan, PhD (ArticulateScience LLC), for medical editorial assistance with this manuscript. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. This study was sponsored and funded by Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare the following relationships: A Hochhaus: reports honoraria from Novartis, BMS, ARIAD and Pfizer; consulting or advisory role for Novartis, BMS, ARIAD and Pfizer; research funding from Novartis, BMS, ARIAD, Pfizer and MSD; travel, accommodation or other expenses paid or reimbursed by Novartis and BMS; T Masszi: reports honoraria from Novartis, Takeda, Janssen-Cilag and BMS; consulting or advisory role for Novartis, Takeda, Janssen-Cilag and BMS; FJ Giles: reports honoraria from Novartis; consulting or advisory role for Novartis; research funding from Novartis; JP Radich: reports consulting or advisory role for Novartis, ARIAD, BMS and Gilead; research funding from Novartis; DM Ross: reports honoraria from Novartis and BMS; consulting or advisory role for Novartis; research funding from Novartis and Celgene; MT Gómez Casares: reports honoraria from BMS, Novarti, and ARIAD; consulting or advisory role for BMS, Novartis and ARIAD; travel, accommodation or other expenses paid or reimbursed by BMS, Novartis and ARIAD; A Hellmann: reports honoraria from Novartis, BMS and ARIAD; travel, accommodation, or other expenses paid or reimbursed by Novartis, Orphan, Servier and BMD; J Stentoft: reports research funding from Novartis, BMS, Pfizer and ARIAD; E Conneally: reports honoraria from Novartis, BMS, Pfizer and Gilead; consulting or advisory role for Novartis, BMS, Pfizer and Gilead; research funding from BMS and Pfizer; V García-Gutiérrez: reports honoraria from Novartis, BMS, Pfizer and ARIAD; consulting or advisory role for Novartis, BMS, Pfizer and ARIAD; research funding from Novartis, BMS, Pfizer and ARIAD; travel, accommodation, or other expenses paid or reimbursed by Novartis, BMS, Pfizer and ARIAD; N Gattermann: reports honoraria, research funding and travel, accommodation, or other expenses paid or reimbursed by Novartis; W Wiktor-Jedrzejczak: reports consulting or advisory role for Roche, Janssen-Cilag, Onconova, Celgene, Sandoz and Angelini; research funding from Amgen, BMS, Novartis and AbbVie; expert testimony for Celgene; travel, accommodation or other expenses paid or reimbursed by Pierre Fabre, Novartis, Roche and Sanofi; PD le Coutre: reports participation in a speakers bureau for Novartis, BMS, Pfizer and ARIAD; B Martino: has nothing to disclose; S Saussele: reports honoraria from Novartis, BMS, Pfizer and ARIAD; consulting or advisory role for Novartis, BMS, Pfizer and ARIAD; research funding from Novartis and BMS; travel, accommodation or other expenses paid or reimbursed by Novartis and BMS; HD Menssen: is employed by, and owns stock in, Novartis Pharma AG; W Deng: is employed by Novartis Pharmaceuticals Corporation; N Krunic: is employed by, and owns stock in, Novartis Institute for Biomedical Research; V Bedoucha: is employed by Novartis Pharma AG; G Saglio: reports consulting or advisory role for Novartis, BMS, ARIAD, and Pfizer.
Additional information
Presented in part at the 2016 American Society of Clinical Oncology Annual Meeting, 3–7 June 2016, Chicago, IL; the 21st European Hematology Association Congress, 9–12 June 2016, Copenhagen, Denmark; the 4th Society of Hematologic Oncology Annual Meeting, 7–10 September 2016, Houston, TX; and the 58th Annual Meeting and Exposition of the American Society of Hematology, 3–6 December 2016, San Diego, CA.
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Hochhaus, A., Masszi, T., Giles, F. et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia 31, 1525–1531 (2017). https://doi.org/10.1038/leu.2017.63
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.63
This article is cited by
-
Measurable residual disease (MRD)-testing in haematological and solid cancers
Leukemia (2024)
-
Management and outcome of patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era – analysis of the European LeukemiaNet Blast Phase Registry
Leukemia (2024)
-
Treatment-free remission after a second TKI discontinuation attempt in patients with Chronic Myeloid Leukemia re-treated with dasatinib – interim results from the DAstop2 trial
Leukemia (2024)
-
Oral arsenic plus imatinib versus imatinib solely for newly diagnosed chronic myeloid leukemia: a randomized phase 3 trial with 5-year outcomes
Journal of Cancer Research and Clinical Oncology (2024)
-
Management of chronic myeloid leukemia in 2023 – common ground and common sense
Blood Cancer Journal (2023)