Abstract
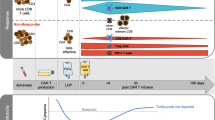
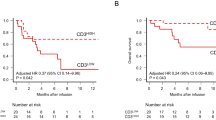
It is unknown, why only a minority of chronic myeloid leukemia (CML) patients sustains treatment free remission (TFR) after discontinuation of tyrosine kinase inhibitor (TKI) therapy in deep molecular remission (MR). Here we studied, whether expression of the T-cell inhibitory receptor (CTLA-4)-ligand CD86 (B7.2) on plasmacytoid dendritic cells (pDC) affects relapse risk after TKI cessation. CML patients in MR displayed significantly higher CD86+pDC frequencies than normal donors (P<0.0024), whereas TFR patients had consistently low CD86+pDC (n=12). This suggested that low CD86+pDC might be predictive of TFR. Indeed, in a prospective analysis of 122 patients discontinuing their TKI within the EURO-SKI trial, the one-year relapse-free survival (RFS) was 30.1% (95% CI 15.6–47.9) for patients with >95 CD86+pDC per 105 lymphocytes, but 70.0% (95% CI 59.3–78.3) for patients with <95 CD86+pDC (hazard ratio (HR) 3.4, 95%-CI: 1.9–6.0; P<0.0001). Moreover, only patients with <95 CD86+pDC derived a significant benefit from longer (>8 years) TKI exposure before discontinuation (HR 0.3, 95% CI 0.1–0.8; P=0.0263). High CD86+pDC counts significantly correlated with leukemia-specific CD8+ T-cell exhaustion (Spearman correlation: 0.74, 95%-CI: 0.21–0.92; P=0.0098). Our data demonstrate that CML patients with high CD86+pDC counts have a higher risk of relapse after TKI discontinuation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hehlmann R, Müller MC, Lauseker M, Hanfstein B, Fabarius A, Schreiber A et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-Study IV. J Clin Oncol 2013; 32: 415–423.
Jabbour E, Kantarjian HM, Saglio G, Steegmann JL, Shah NP, Boqué C et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014; 123: 494–500.
Larson RA, Hochhaus A, Hughes TP, Clark RE, Etienne G, Kim DW et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia 2012; 26: 2197–2203.
Graham SM, Jørgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002; 99: 319–325.
O'Hare T, Zabriskie MS, Eiring AM, Deininger MW . Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat Rev Cancer 2012; 12: 513–526.
Branford S, Yeung DT, Ross DM, Prime JA, Field CR, Altamura HK et al. Early molecular response and female sex strongly predict stable undetectable BCR-ABL1, the criteria for imatinib discontinuation in patients with CML. Blood 2013; 121: 3818–3824.
Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med 2003; 349: 1423–1432.
Cortes J, O'Brien S, Kantarjian H . Discontinuation of imatinib therapy after achieving a molecular response. Blood 2004; 104: 2204–2205.
Rousselot P, Huguet F, Réa D, Legros L, Cayuela JM, Maarek O et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood 2007; 109: 58–60.
Mauro MJ, Druker BJ, Maziarz RT . Divergent clinical outcome in two CML patients who discontinued imatinib therapy after achieving a molecular remission. Leukemia Res 2004; 28 (Suppl 1): S71–S73.
Merante S, Orlandi E, Bernasconi P, Calatroni S, Boni M, Lazzarino M . Outcome of four patients with chronic myeloid leukemia after imatinib mesylate discontinuation. Haematologica 2005; 90: 979–981.
Mahon F-X, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010; 11: 1029–1035.
Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol 2013; 32: 424–430.
Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 2013; 122: 515–522.
Cross NCP, White HE, Müller MC, Saglio G, Hochhaus A . Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia 2012; 26: 2172–2175.
Müller MC, Cross NCP, Erben P, Schenk T, Hanfstein B, Ernst T et al. Harmonization of molecular monitoring of CML therapy in Europe. Leukemia 2009; 23: 1957–1963.
Cross NCP, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia 2015; 29: 999–1003.
Ross DM, Hughes TP . How I determine if and when to recommend stopping tyrosine kinase inhibitor treatment for chronic myeloid leukaemia. Br J Haematol 2014; 166: 3–11.
Ilander M, Hekim C, Mustjoki S . Immunology and immunotherapy of chronic myeloid leukemia. Curr Hematol Malig Rep 2014; 9: 17–23.
Mahon FX, Delbrel X, Cony-Makhoul P, Fabères C, Boiron JM, Barthe C et al. Follow-up of complete cytogenetic remission in patients with chronic myeloid leukemia after cessation of interferon alfa. J Clin Oncol 2002; 20: 214–220.
Bonifazi F, de Vivo A, Rosti G, Guilhot F, Guilhot J, Trabacchi E et al. Chronic myeloid leukemia and interferon-alpha: a study of complete cytogenetic responders. Blood 2001; 98: 3074–3081.
Hochhaus A, Reiter A, Saußele S, Reichert A, Emig M, Kaeda J et al. Molecular heterogeneity in complete cytogenetic responders after interferon-alpha therapy for chronic myelogenous leukemia: low levels of minimal residual disease are associated with continuing remission. German CML Study Group and the UK MRC CML Study Group. Blood 2000; 95: 62–66.
Talpaz M, Estrov Z, Kantarjian H, Ku S, Foteh A, Kurzrock R . Persistence of dormant leukemic progenitors during interferon-induced remission in chronic myelogenous leukemia. Analysis by polymerase chain reaction of individual colonies. J Clin Invest 1994; 94: 1383–1389.
Kantarjian HM, O'Brien S, Cortes JE, Shan J, Giles FJ, Rios MB et al. Complete cytogenetic and molecular responses to interferon-alpha-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer 2003; 97: 1033–1041.
Burchert A, Saußele S, Eigendorff E, Müller MC, Sohlbach K, Inselmann S et al. Interferon alpha 2 (IFN) maintenance therapy may enable high rates of treatment discontinuation in chronic myeloid leukemia (CML). Leukemia 2015; 29: 1331–1335.
Talpaz M, Kantarjian HM, McCredie KB, Keating MJ, Trujillo J, Gutterman J . Clinical investigation of human alpha interferon in chronic myelogenous leukemia. Blood 1987; 69: 1280–1288.
Talpaz M, Hehlmann R, Quintás-Cardama A, Mercer J, Cortes J . Re-emergence of interferon-α in the treatment of chronic myeloid leukemia. Leukemia 2013; 27: 803–812.
Crouse J, Kalinke U, Oxenius A . Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol 2015; 15: 231–242.
Molldrem JJ, Lee PP, Wang C, Felio K, Kantarjian HM, Champlin RE et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med 2000; 6: 1018–1023.
Burchert A, Wölfl S, Schmidt M, Brendel C, Denecke B, Cai D et al. Interferon-alpha, but not the ABL-kinase inhibitor imatinib (STI571), induces expression of myeloblastin and a specific T-cell response in chronic myeloid leukemia. Blood 2003; 101: 259–264.
Burchert A, Müller MC, Kostrewa P, Erben P, Bostel T, Liebler S et al. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J Clin Oncol 2010; 28: 1429–1435.
Chen L . Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004; 4: 336–347.
Pardoll DM . The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264.
Francisco LM, Sage PT, Sharpe AH . The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236: 219–242.
Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, Dezutter-Dambuyant C et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat Immunol 2001; 2: 1144–1150.
Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M . Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nat Rev Immunol 2011; 11: 558–565.
Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity 2008; 29: 464–475.
Villadangos JA, Young L . Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 2008; 29: 352–361.
Gilliet M, Liu Y-J . Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med 2002; 195: 695–704.
Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Terry M. Therneau, (Springer. springer.com, 2000). Available at:http://www.springer.com/us/book/9780387987842 (Accessed 16 April 2016).
Altman DG, Lausen B, Sauerbrei W, Schumacher M . Dangers of using ‘optimal’ cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994; 86: 829–835.
Hothorn T, Hornik K, Zeileis A. Unbiades Recursive Partitioning: A Conditional Interfernce Framework. statmath.wu-wien.ac.at. 2006. Available at: http://statmath.wu-wien.ac.at/~zeileis/papers/Hothorn+Hornik+Zeileis-2006.pdf (Accessed 16 April 2016).
Davison AC, Hinkley DV. Bootstrap Methods and Their Application. statwww.epfl.ch. Available at: http://statwww.epfl.ch/davison/BMA/CUPsample.pdf (Accessed 16 April 2016).
Silverman BW. Density estimation for statistics and data analysis. ned.ipac.caltech.edu. Available at: https://ned.ipac.caltech.edu/level5/March02/Silverman/paper.pdf (Accessed 16 April 2016).
Altman DG, JM B. Diagnostic tests. 1: Sensitivity and specificity. - PubMed - NCBI. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8019315.
Diagnostic tests 2: Predictive values. - PubMed - NCBI. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8038641.
Mahon F-X, Richter J, Guilhot J, Müller MC, Dietz C, Kimmoo P et al. Interim Analysis of a Pan European Stop Tyrosine Kinase Inhibitor Trial in Chronic Myeloid Leukemia : The EURO-SKI study. Blood 2014; 124: 151.
Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu Y-J et al. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol 2004; 173: 4433–4442.
Sawant A, Hensel JA, Chanda D, Harris BA, Siegal GP, Maheshwari A et al. Depletion of plasmacytoid dendritic cells inhibits tumor growth and prevents bone metastasis of breast cancer cells. J Immunol 2012; 189: 4258–4265.
Molldrem JJ, Lee PP, Kant S, Wieder E, Jiang W, Lu S et al. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest 2003; 111: 639–647.
Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 2006; 7: 652–662.
Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK . Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity 1997; 6: 411–417.
Ueda H, Howson JMM, Esposito L, Heward J, Snook H, Chamberlain G et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 2003; 423: 506–511.
Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000; 12: 431–440.
Ilander M, Olsson-Strömberg U, Schlums H, Guilhot J, Brück O, Lähteenmäki H et al. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia 2016; e-pub ahead of print 16 December 2016 doi:10.1038/leu.2016.360.
Vasaturo A, Di Blasio S, Peeters DGA, de Koning CCH, de Vries JM, Figdor CG et al. Clinical implications of co-inhibitory molecule expression in the tumor microenvironment for DC vaccination: a game of stop and Go. Front Immunol 2013; 4: 417.
Acknowledgements
This work was supported by the Clinical Research Group - KFO210 - of the German Research Foundation (DFG), and by the German José Carreras Leukaemia Foundation (DJCLS, R14/07 and AH 06-01).
Author contributions
AB designed the study, recruited and treated patients, interpreted the data and wrote the manuscript. CS processed samples, acquired the analytical data, analyzed the results, and helped generating figures and writing the manuscript. MP did the statistical analysis, interpreted the data, generated figures and helped in writing the manuscript. JG acquired and processed the clinical data and contributed to the statistical analysis. FF contributed to the statistical analysis. SS and FXM were the principal investigators of the EURO-SKI trial, SS recruited and treated patients of this sub-study. MM, EE, THB, CW, JD, MEG, RH, GF, SH, TI, YW, TL, AH recruited and treated patients. SI, CTD, CAB, YW, MH, RH, AN were involved in data acquisition and interpretation. All authors interpreted the data, drafted and reviewed the report, gave their final approval for publication, and agreed to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
AB reports personal fees from Bristol-Myers Squibb, outside the submitted work. SS, AH, FXM, MM report honoraria, travel support and research support from Novartis, Bristol-Myers Squibb, Ariad and Pfizer, outside the submitted work.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Rights and permissions
About this article
Cite this article
Schütz, C., Inselmann, S., Sausslele, S. et al. Expression of the CTLA-4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia 31, 829–836 (2017). https://doi.org/10.1038/leu.2017.9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2017.9
This article is cited by
-
Switching from imatinib to nilotinib plus pegylated interferon-α2b in chronic phase CML failing to achieve deep molecular response: clinical and immunological effects
Annals of Hematology (2023)
-
Discontinuation of Tyrosine Kinase Inhibitors in Patients with Chronic Myeloid Leukemia: a Review of the Biological Factors Associated with Treatment-Free Remission
Current Oncology Reports (2022)
-
Therapy Resistance and Disease Progression in CML: Mechanistic Links and Therapeutic Strategies
Current Hematologic Malignancy Reports (2022)
-
Improving outcomes in chronic myeloid leukemia through harnessing the immunological landscape
Leukemia (2021)
-
Treatment-free remission and immunity in chronic myeloid leukemia
International Journal of Hematology (2021)