Abstract
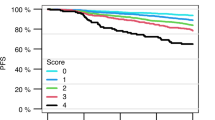
The introduction of tyrosine kinase inhibitors (TKI) in the treatment of Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) has revolutionized the outcome, but the prognosis of the disease is still based on prognostic systems that were developed in the era of conventional chemotherapy and interferon (IFN)-alfa. A new prognostic score including only two variables, spleen size and basophils, was developed for the prediction of complete cytogenetic response (CCyR) and progression-free survival (PFS). The score was based on a large series of patients who were enrolled in prospective multicenter studies of first-line imatinib treatment. The prognostic value of the EUTOS (European Treatment and Outcome Study for CML) score has now been tested in an independent, multicenter, multinational series of 1288 patients who were treated first-line with imatinib outside prospective studies. It was found that also in these patients, the EUTOS prognostic score was predictive for CCyR, PFS and overall survival (OS). In addition, the prognostic value of the score was reported to be significant in seven of the eight other independent studies of almost 2000 patients that were performed in Europe, the Americas and Asia. The EUTOS risk score is a valid tool for the prediction of the therapeutic effects of TKI, particularly imatinib.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Quintas-Cardama A, Cortes J . Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood 2009; 113: 1619–1630.
Hehlmann R, Hochhaus A, Baccarani M . Chronic myeloid leukaemia. Lancet 2007; 370: 342–350.
Silver RT, Woolf SH, Hehlmann R, Appelbaum FR, Anderson J, Bennett C et al. An evidence-based analysis of the effect of busulfan, hydroxyurea, interferon and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American Society of Hematology. Blood 1999; 94: 1517–1536.
Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE et al. Prognostic discrimination in "good-risk" chronic granulocytic leukemia. Blood 1984; 63: 789–799.
Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst 1998; 90: 850–858.
Deininger M, Buchdunger E, Druker BJ . The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 2005; 105: 2640–2653.
Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of European LeukemiaNet. Blood 2006; 108: 1809–1820.
Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009; 27: 6041–6051.
Kantarjian H, O’Brien S, Jabbour E, Garcia-Manero G, Quintas-cardama A, Shan J et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: a single institution historical experience. Blood 2012; 119: 1981–1987.
Hughes T, Kaeda J, Branford S, Rudki Z, Hochhaus A, Hensley ML et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnoses chronic myeloid leukemia. N Engl J Med 2003; 349: 1423–1432.
Rosti G, Trabacchi E, Bassi S, Bonifazi F, De Vivo A, Martinelli G et al. Risk and early cytogenetic response to imatinib and interferon in chronic myeloid leukemia. Haematologica 2003; 88: 256–259.
Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006; 355: 2408–2417.
De Lavallade H, Apperley JF, Khorashad J, Milojkovic D, Reid AG, Bua M et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained response in an intention-to-treat analysis. J Clin Oncol 2008; 26: 3358–3363.
Hochhaus A, O’Brien SG, Guilhot F, Druker BJ, Branford S, Foroni L et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009; 23: 1054–1061.
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259.
Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M et al. Dasatinib versus imatinib in newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–2270.
Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood 2011; 118: 686–692.
Guilhot J, Baccarani M, Clark RE, Cervantes F, Guilhot F, Hochhaus A et al. Definitions, methodological and statistical issues for phase 3 clinical trials in chronic myeloid leukemia: a proposal by European LeukemiaNet. Blood 2012; 119: 5963–5971.
Choudhury JB . Non-parametric confidence interval estimation for competing risks analysis: application to contraceptive data. Stat Med 2002; 21: 1129–1144.
Pfirrmann M, Hochhaus A, Lauseker M, Saussele S, Hehlmann R, Hasford J . Recommendations to meet statistical challenges arising from endpoints beyond overall survival in clinical trials on chronic myeloid leukemia. Leukemia 2011; 25: 1433–1438.
Mahon F-X, Belloc F, Lagarde V, Chollet C, Moreau-Gaudry F, Reiffers J et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood 2003; 101: 2368–2373.
Illmer T, Schaich M, Platzbecker U, Freiberg-Richter J, Oelschlagel U, von Bonin M et al. P-Glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia 2004; 18: 401–408.
Raaijmakers MHGP . ATP-binding-cassette transporters in hematopoietic stem cells and their utility as therapeutical targets in acute and chronic myeloid leukemia. Leukemia 2007; 21: 2094–2102.
Dulucq S, Bouchet S, Turcq B, Lippert E, Etienne G, Reiffers J et al. Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 2008; 112: 2024–2027.
Angelini S, Soverini S, Ravegnini G, Barnett M, Turrini E, Thornquist M et al. Association between imatinib transporters and metabolizing enzymes genotype and response in newly diagnosed chronic myeloid leukemia patients receiving imatinib therapy. Haematologica 2013; 98: 192–200.
Thomas J, Wang L, Clark RE, Pirmohamed M . Active transport of imatinib into and out of cells: implications for drug resistance. Blood 2004; 104: 3739–3745.
White DL, Dang P, Engler J, Frede A, Zrim S, Osborn M et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol 2010; 28: 2761–2767.
White DL, Saunders VA, Dang P, Engler J, Venables A, Zrim S et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood 2007; 110: 4064–4072.
White DL, Radich J, Soverini S, Saunders VA, Frede A, Dang P et al. Chronic phase chronic myeloid leukemia patients with low OCT-1 activity randomised to high-dose imatinib achieve better responses, and lower failure rates, than those randomized to standard-dose. Haematologica 2012; 97: 907–914.
Ng KP, Hillmer AM, Chuah CTH, Juan WC, Ko TK, Teo ASM et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med 2012; 18: 521–528.
Mahon F-X, Augis V, Airiau K, Josselin M, Turc B, Belloc F . A single nucleotide polymorphism in the coding sequence of BIM is associated with poor prognostic in chronic myeloid leukemia treated by imatinib. Blood 2012; 120, (ASH abstract no. 1683).
Lucas CM, Harris RJ, Giannoudis A, Davies A, Knight K, Watmough SJ et al. Chronic myeloid leukemia patients with the e13a2 BCR-ABL fusion transcript have inferior responses to imatinib compared to patients with the e14a2 transcript. Haematologica 2009; 94: 1362–1367.
Castagnetti F, Gugliotta G, Palandri F, Breccia M, Stagno F, Levato L et al. The BCR-ABL1 transcript type does not influence the response and the outcome of chronic myeloid leukemia patients treated frontline with nilotinib. Blood 2012; 120, (ASH abstract no. 1680).
Romo CG, Kantarjian HM, Luthra R, Quintas-Cardama A, Jabbour EJ, Borthakur J et al. Response to frontline therapy with second generation tyrosine kinase inhibitors in chronic myeloid leukemia: analysis of outcome for b3a2 vs b2a2 fusion transcripts. Blood 2012; 120, (ASH abstract no. 3781).
Castagnetti F, Breccia M, Levato L, Cortelezzi A, Abruzzese E, Albano F et al. B2A2 BCR-ABL fusion transcript is a candidate adverse prognostic factor in chronic myeloid leukemia patients treated frontline with imatinib mesylate. Haematologica 2011; 96: 205 (EHA abstract no. 0487).
Khorashad JS, Wagner S, Greener L, Marin D, Reid A, Milojkovic D et al. The level of BCR-ABL1 kinase activity before treatment does not identify chronic myeloid leukemia patients who fail to achieve a complete cytogenetic response on imatinib. Haematologica 2009; 94: 861–864.
Vigneri PG, Stagno F, Stella S, Cupri A, Forte S, Massimino M et al. High BCR-ABL levels at diagnosis are associated with unfavourable responses to imatinib mesylate. Blood 2012; 120, (ASH abstract no. 2790).
Marzocchi G, Castagnetti F, Luatti S, Baldazzi C, Stacchini M, Gugliotta G et al. Variant Philadelphia translocations: molecular-cytogenetic characterization and prognostic influence on frontline imatinib therapy, a GIMEMA Working Party on CML analysis. Blood 2011; 117: 6793–6800.
Fabarius A, Leitner A, Hochhaus A, Muller MC, Hanfstein B, Haferlach C et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood 2011; 118: 6760–6768.
Luatti S, Castagnetti F, Marzocchi G, Baldazzi C, Gugliotta G, Iacobucci I et al. Additional chromosome abnormalities in Philadelphia-positive clone: adverse prognostic influence on frontline imatinib therapy: a GIMEMA Working Party on CML analysis. Blood 2012; 120: 761–767.
Sokal JE, Baccarani M, Russo D . Staging and prognosis in chronic myelogenous leukemia. Semin Hematol 1988; 25: 49–61.
Kantarjian HM, Dixon D, Keating MJ . Characteristics of accelerated disease in chronic myelogenous leukemia. Cancer 1988; 61: 1141–1146.
Muller-Berat CN, Wantzin GL, Philip P, Baccarani M, Killmann S-A . Agar culture studies of bone marrow, blood, spleen, and liver in chronic myeloid leukemia. Leuk Res 1977; 1: 123–131.
Schemionek M, Spieker T, Kerstiens L, Elling C, Essers M, Trumpp A et al. Leukemic spleen cells are more potent than bone marrow-derived cells in a transgenic mouse model of CML. Leukemia 2011; 26: 1030–1037.
Baccarani M, Zaccaria A, Santucci MA, Bagnara GP, Ricci P, Gobbi M et al. A simultaneous study of bone marrow, spleen and liver in chronic myeloid leukemia: evidence for differences in cell composition and karyotypes. Ser Haematol 1975; 8: 81–112.
Baccarani M, Hasford J, Hoffmann V, Guilhot J, Saussele S, Rosti G et al. A new prognostic score (EUTOS score) predicting cytogenetic response and progression-free survival in 2060 patients with chronic myeloid leukemia on imatinib treatment. Haematologica 2011; 96: 422 (EHA abstract no. 1010).
Marin D, Ibrahim AR, Goldman J . European treatment and outcome study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol 2011; 29: 3944–3955.
Breccia M, Finsinger P, Loglisci G, Latagliata R, Mancini M, Salaroli A et al. The EUTOS score identifies chronic myeloid leukaemia patients with poor prognosis treated with imatinib first or second line. Leuk Res 2012; 36: e209–e210.
Pagnano KB, Lorand-Metze I, Miranda ECM, O Duarte V, Delamain MT, O Duarte G et al. EUTOS score is predictive of event-free survival, but not for progression-free and overall survival in patients with early chronic phase chronic myeloid leukemia treated with imatinib: a single Institution experience. Blood 2012; 120, (ASH abstract no. 1681).
Yahng S-A, Jang E-J, Choi SY, Bang J-H, Park J-E, Leon H-L et al. Comparison of Sokal, Hasford and EUTOS scores in term of long-term treatment outcome according to the risks in each prognostic model: a single center data analysed in 255 early chronic phase chronic myeloid leukemia patients treated with frontline imatinib mesylate. Blood 2012; 120, (ASH abstract no. 2794).
Than H, Kuan L, Seow CH, Li W, Allen JC, Chuah C . The EUTOS score is highly predictive for clinical outcome and survival in Asian patients with early chronic phase chronic myeloid leukemia treated with imatinib. Blood 2012; 120, (ASH abstract 3758).
Tiribelli M, Bonifacio M, Calistri E, Binotto G, Maino E, Marin L et al. EUTOS score identifies cases with poor outcome in patients with early phase chronic myeloid leukemia though not predictive for optimal response to imatinib. Blood 2012; 120, (ASH abstract 3778).
Jabbour E, Cortes J, Nazha A, O’Brien S, Quintas-Cardama A, Pierce S et al. EUTOS score is not predictive for survival and outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors: a single institution experience. Blood 2012; 119: 4524–4526.
Saussele S, Lauseker M, Hoffmann V, Lindörfer D, Hanfstein B, Proetel U et al. The EUTOS high-risk population differs substantially from the EURO score high-risk group; the EUTOS score predicts molecular response in chronic phase CML patients: results of the German CML-Study IV. Haematologica 2012; 97 (s1): 454 EHA abstract no 1105.
Castagnetti F, Gugliotta G, Palandri F, Breccia M, Levato L, Stagno F et al. EUTOS score is predictive for survival and outcome in patients with early chronic phase chronic myeloid leukemia treated with nilotinib-based regimens. Haematologica 2012; 97: 76–77, (EHA abstract no. 193).
McWeeney S, Pemberton LC, Loriaux MM, Vartanian K, Willis SG, Yochum G et al. A gene espression signature of CD34+ cells to predict major cytogenetic response on chronic-phase chronic myeloid leukemia patients treated with imatinib. Blood 2010; 115: 315–325.
Jiang X, Forrest D, Nicolini F, Turhan A, Guilhot J, Yip C et al. Properties of CD34+ CML stem/progenitor cells that correlate with different clinical responses to imatinib mesylate. Blood 2010; 116: 2112–2121.
Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM et al. Assessmentof BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol 2012; 30: 232–238.
Hanfstein B, Muller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia. Leukemia 2012; 26: 2096–2102.
Hoffmann V, Baccarani M, Hasford J, Guilhot J, Saussele S, Rosti G et al. The EUTOS CML score aims to support clinical decision-making. Blood 2012; 119: 2966–2967.
Acknowledgements
The European Treatment and Outcome Study (EUTOS) is a project supported by the Novartis Oncology Europe through a contract with European LeukemiaNet and the University of Heidelberg (Germany).
Author Contributions
MB, VH (principal investigator) and MP designed the research. MB, FC, AT, AZ, AH, WP, J-LS JM, KI AC and GR collected the data. VH performed the statistical analysis. DL processed and analyzed the data. VH, MB and MP analyzed and interpreted the data and wrote the manuscript. All authors revised and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
VH (principal investigator) received funding from Novartis Pharma, and honoraria from Bristol Myers Squibb. MB and DL receive research funding from Novartis Pharma. FC has the role of a consultant, and received honoraria from Novartis Pharma and Bristol Myers Squibb. AT has the role of a consultant in Bristol Myers Squibb and Novartis Pharma. AZ received research funding from the University of Heidelberg. J-LS has the role of a consultant, received research grants and honoraria from Novartis Pharma, Bristol Myers Squibb and Pfizer. GR received honoraria from Novartis Pharma, Bristol Myers Squibb, Pfizer and Roche, and holds a consultancy role with Novartis Pharma and Bristol Myers Squibb. MP has the role of a consultant in Novartis Pharma and received honoraria from Bristol Myers Squibb. The remaining authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hoffmann, V., Baccarani, M., Lindoerfer, D. et al. The EUTOS prognostic score: review and validation in 1288 patients with CML treated frontline with imatinib. Leukemia 27, 2016–2022 (2013). https://doi.org/10.1038/leu.2013.171
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2013.171
Keywords
This article is cited by
-
Imatinib and Patient-Related Outcomes in Chronic Myeloid Leukemia: A Single Centric Experience
SN Comprehensive Clinical Medicine (2022)
-
The EUTOS long-term survival (ELTS) score is superior to the Sokal score for predicting survival in chronic myeloid leukemia
Leukemia (2020)
-
Defining therapy goals for major molecular remission in chronic myeloid leukemia: results of the randomized CML Study IV
Leukemia (2018)
-
Performance of Sokal and Eutos Scores for Predicting Cytogenetic and Molecular Response in Newly Diagnosed Chronic Myeloid Leukemia-Chronic Phase Patients on Imatinib
Indian Journal of Hematology and Blood Transfusion (2017)
-
Treatment and outcome of 2904 CML patients from the EUTOS population-based registry
Leukemia (2017)