Abstract
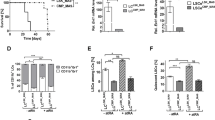
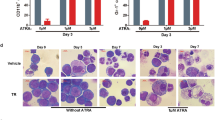
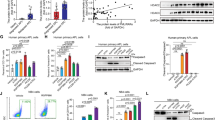
All-trans retinoic acid (ATRA) is the only clinically useful differentiating agent, being used in the treatment of acute promyelocytic leukemia (APL). The use of ATRA in other types of acute myelogenous leukemia (AML) calls for the identification of novel strategies aimed at increasing its therapeutic activity. Here, we provide evidence that pharmacological inhibition of the mitogen-activated protein kinase, p38α, or silencing of the corresponding gene sensitizes APL and AML cell lines, as well as primary cultures of AML blasts to the anti-proliferative and cyto-differentiating activity of ATRA and synthetic retinoids. P38α inhibits ligand-dependent transactivation of the nuclear retinoic acid receptor, RARα, and the derived chimeric protein expressed in the majority of APL cases, PML-RARα. Inhibition is the consequence of ligand-independent binding of p38α, which results in stabilization of RARα and PML-RARα via blockade of their constitutive degradation by the proteasome. The inhibitory effect requires a catalytically active p38α and direct physical interaction with RARα and PML-RARα. Ser-369 in the E-region of RARα is essential for the binding of p38α and the ensuing functional effects on the activity of the receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kelaidi C, Chevret S, De Botton S, Raffoux E, Guerci A, Thomas X et al. Improved outcome of acute promyelocytic leukemia with high WBC counts over the last 15 years: the european APL group experience. J Clin Oncol 2009; 27: 2668–2676.
Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, Estey EH et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the european LeukemiaNet. Blood 2009; 113: 1875–1891.
Sanz MA, Lo-Coco F . Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol 2011; 29: 495–503.
Sanz MA, Vellenga E, Rayon C, Diaz-Mediavilla J, Rivas C, Amutio E et al. All-trans retinoic acid and anthracycline monochemotherapy for the treatment of elderly patients with acute promyelocytic leukemia. Blood 2004; 104: 3490–3493.
Tallman MS . Differentiating therapy in acute myeloid leukemia. Leukemia 1996; 10: 1262–1268.
Tallman MS . Acute promyelocytic leukemia as a paradigm for targeted therapy. Semin Hematol 2004; 41: 27–32.
Tallman MS, Nabhan C, Feusner JH, Rowe JM . Acute promyelocytic leukemia: evolving therapeutic strategies. Blood 2002; 99: 759–767.
Ablain J, de The H . Revisiting the differentiation paradigm in acute promyelocytic leukemia. Blood 2011; 117: 5795–5882.
Gianni M, Li Calzi M, Terao M, Guiso G, Caccia S, Barbui T et al. AM580, a stable benzoic derivative of retinoic acid, has powerful and selective cyto-differentiating effects on acute promyelocytic leukemia cells. Blood 1996; 87: 1520–1531.
Nasr R, Guillemin MC, Ferhi O, Soilihi H, Peres L, Berthier C et al. Eradication of acute promyelocytic leukemia-initiating cells through PML-RARA degradation. Nat Med 2008; 14: 1333–1342.
Garattini E, Gianni M, Terao M . Retinoids as differentiating agents in oncology: a network of interactions with intracellular pathways as the basis for rational therapeutic combinations. Curr Pharm Des 2007; 13: 1375–1400.
Garattini E, Gianni M, Terao M . Cytodifferentiation by retinoids, a novel therapeutic option in oncology: rational combinations with other therapeutic agents. Vitam Horm 2007; 75: 301–354.
Cassinat B, Zassadowski F, Ferry C, Llopis L, Bruck N, Lainey E et al. New role for granulocyte colony-stimulating factor-induced extracellular signal-regulated kinase 1/2 in histone modification and retinoic acid receptor alpha recruitment to gene promoters: relevance to acute promyelocytic leukemia cell differentiation. Mol Cell Biol 2011; 31: 1409–1418.
Gianni M, Terao M, Norio P, Barbui T, Rambaldi A, Garattini E . All-trans retinoic acid and cyclic adenosine monophosphate cooperate in the expression of leukocyte alkaline phosphatase in acute promyelocytic leukemia cells. Blood 1995; 85: 3619–3635.
Gianni M, Terao M, Zanotta S, Barbui T, Rambaldi A, Garattini E . Retinoic acid and granulocyte colony-stimulating factor synergistically induce leukocyte alkaline phosphatase in acute promyelocytic leukemia cells. Blood 1994; 83: 1909–1921.
Gianni M, Bauer A, Garattini E, Chambon P, Rochette-Egly C . Phosphorylation by p38MAPK and recruitment of SUG-1 are required for RA-induced RAR gamma degradation and transactivation. EMBO J 2002; 21: 3760–3769.
Lalevee S, Bour G, Quinternet M, Samarut E, Kessler P, Vitorino M et al. Vinexinss, an atypical ‘sensor’ of retinoic acid receptor gamma signaling: union and sequestration, separation, and phosphorylation. FASEB J 2010; 24: 4523–4534.
Rochette-Egly C, Germain P . Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs). Nucl Recept Signal 2009; 7: e005.
Gianni M, Parrella E, Raska Jr I, Gaillard E, Nigro EA, Gaudon C et al. P38MAPK-dependent phosphorylation and degradation of SRC-3/AIB1 and RARalpha-mediated transcription. EMBO J 2006; 25: 739–751.
Bruck N, Vitoux D, Ferry C, Duong V, Bauer A, de The H et al. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARalpha to target promoters. EMBO J 2009; 28: 34–47.
Bour G, Lalevee S, Rochette-Egly C . Protein kinases and the proteasome join in the combinatorial control of transcription by nuclear retinoic acid receptors. Trends Cell Biol 2007; 17: 302–309.
Bour G, Gaillard E, Bruck N, Lalevee S, Plassat JL, Busso D et al. Cyclin H binding to the RARalpha activation function (AF)-2 domain directs phosphorylation of the AF-1 domain by cyclin-dependent kinase 7. Proc Natl Acad Sci USA 2005; 102: 16608–16613.
Gianni M, Boldetti A, Guarnaccia V, Rambaldi A, Parrella E, Raska Jr I et al. Inhibition of the peptidyl-prolyl-isomerase Pin1 enhances the responses of acute myeloid leukemia cells to retinoic acid via stabilization of RARalpha and PML-RARalpha. Cancer Res 2009; 69: 1016–1026.
Gianni M, Kalac Y, Ponzanelli I, Rambaldi A, Terao M, Garattini E . Tyrosine kinase inhibitor STI571 potentiates the pharmacologic activity of retinoic acid in acute promyelocytic leukemia cells: effects on the degradation of RARalpha and PML-RARalpha. Blood 2001; 97: 3234–3243.
Gu ZM, Wu YL, Zhou MY, Liu CX, Xu HZ, Yan H et al. Pharicin B stabilizes retinoic acid receptor-alpha and presents synergistic differentiation induction with ATRA in myeloid leukemic cells. Blood 2010; 116: 5289–5297.
Kale VP . Differential activation of MAPK signaling pathways by TGF-beta1 forms the molecular mechanism behind its dose-dependent bidirectional effects on hematopoiesis. Stem Cells Dev 2004; 13: 27–38.
Kale VP, Vaidya AA . Molecular mechanisms behind the dose-dependent differential activation of MAPK pathways induced by transforming growth factor-beta1 in hematopoietic cells. Stem Cells Dev 2004; 13: 536–547.
Gaillard E, Bruck N, Brelivet Y, Bour G, Lalevee S, Bauer A et al. Phosphorylation by PKA potentiates retinoic acid receptor alpha activity by means of increasing interaction with and phosphorylation by cyclin H/cdk7. Proc Natl Acad Sci USA 2006; 103: 9548–9553.
Ota A, Zhang J, Ping P, Han J, Wang Y . Specific regulation of noncanonical p38alpha activation by Hsp90-Cdc37 chaperone complex in cardiomyocyte. Circ Res 2010; 106: 1404–1412.
Zhou H, Zheng M, Chen J, Xie C, Kolatkar AR, Zarubin T et al. Determinants that control the specific interactions between TAB1 and p38alpha. Mol Cell Biol 2006; 26: 3824–3834.
Lanotte M, Martin-Thouvenin V, Najman S, Balerini P, Valensi F, Berger R . NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 1991; 77: 1080–1086.
Gallagher RE, Bilello PA, Ferrari AC, Chang CS, Yen RW, Nickols WA et al. Characterization of differentiation-inducer-resistant HL-60 cells. Leuk Res 1985; 9: 967–986.
Quentmeier H, Martelli MP, Dirks WG, Bolli N, Liso A, Macleod RA et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia 2005; 19: 1760–1767.
Gaub MP, Rochette-Egly C, Lutz Y, Ali S, Matthes H, Scheuer I et al. Immunodetection of multiple species of retinoic acid receptor alpha: evidence for phosphorylation. Exp Cell Res 1992; 201: 335–346.
Ferry C, Gianni M, Lalevee S, Bruck N, Plassat JL, Raska Jr I et al. SUG-1 plays proteolytic and non-proteolytic roles in the control of retinoic acid target genes via its interaction with SRC-3. J Biol Chem 2009; 284: 8127–8135.
Gianni M, Ponzanelli I, Mologni L, Reichert U, Rambaldi A, Terao M et al. Retinoid-dependent growth inhibition, differentiation and apoptosis in acute promyelocytic leukemia cells. Expression and activation of caspases. Cell Death Differ 2000; 7: 447–460.
Clerk A, Sugden PH . The p38-MAPK inhibitor, SB203580, inhibits cardiac stress-activated protein kinases/c-Jun N-terminal kinases (SAPKs/JNKs). FEBS Lett 1998; 426: 93–96.
Dermime S, Grignani F, Rogaia D, Liberatore C, Marchesi E, Gambacorti-Passerini C . Acute promyelocytic leukaemia cells resistant to retinoic acid show further perturbation of the RAR alpha signal transduction system. Leuk Lymphoma 1995; 16: 289–295.
Gianni M, Zanotta S, Terao M, Garattini S, Garattini E . Effects of synthetic retinoids and retinoic acid isomers on the expression of alkaline phosphatase in F9 teratocarcinoma cells. Biochem Biophys Res Commun 1993; 196: 252–259.
Alvarez S, Khanwalkar H, Alvarez R, Erb C, Martinez C, Rodriguez-Barrios F et al. C3 halogen and c8’ substituents on stilbene arotinoids modulate retinoic Acid receptor subtype function. ChemMedChem 2009; 4: 1630–1640.
Idres N, Marill J, Chabot GG . Regulation of CYP26A1 expression by selective RAR and RXR agonists in human NB4 promyelocytic leukemia cells. Biochem Pharmacol 2005; 69: 1595–1601.
Balmer JE, Blomhoff R . Gene expression regulation by retinoic acid. J Lipid Res 2002; 43: 1773–1808.
Matt N, Ghyselinck NB, Wendling O, Chambon P, Mark M . Retinoic acid-induced developmental defects are mediated by RARbeta/RXR heterodimers in the pharyngeal endoderm. Development 2003; 130: 2083–2093.
Balajthy Z, Csomos K, Vamosi G, Szanto A, Lanotte M, Fesus L . Tissue-transglutaminase contributes to neutrophil granulocyte differentiation and functions. Blood 2006; 108: 2045–2054.
Csomos K, Nemet I, Fesus L, Balajthy Z . Tissue transglutaminase contributes to the all-trans-retinoic acid-induced differentiation syndrome phenotype in the NB4 model of acute promyelocytic leukemia. Blood 2010; 116: 3933–3943.
Platko JD, Forbes ME, Varvayanis S, Williams MN, Brooks III SC, Cherington V et al. Polyoma middle T antigen in HL-60 cells accelerates hematopoietic myeloid and monocytic cell differentiation. Exp Cell Res 1998; 238: 42–50.
Breitman TR, Selonick SE, Collins SJ . Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA 1980; 77: 2936–2940.
Falini B, Albiero E, Bolli N, De Marco MF, Madeo D, Martelli M et al. Aberrant cytoplasmic expression of C-terminal-truncated NPM leukaemic mutant is dictated by tryptophans loss and a new NES motif. Leukemia 2007; 21: 2052–2054, author reply 2054; discussion 2055–2056.
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352: 254–266.
Falini B, Bigerna B, Pucciarini A, Tiacci E, Mecucci C, Morris SW et al. Aberrant subcellular expression of nucleophosmin and NPM-MLF1 fusion protein in acute myeloid leukaemia carrying t(3;5): a comparison with NPMc+ AML. Leukemia 2006; 20: 368–371.
Schlenk RF, Dohner K, Kneba M, Gotze K, Hartmann F, Del Valle F et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG Trial AML HD98B. Haematologica 2009; 94: 54–60.
Balusu R, Fiskus W, Rao R, Chong DG, Nalluri S, Mudunuru U et al. Targeting levels or oligomerization of nucleophosmin 1 induces differentiation and loss of survival of human AML cells with mutant NPM1. Blood 2011; 118: 3096–3106.
Tenen DG . Abnormalities of the CEBP alpha transcription factor: a major target in acute myeloid leukemia. Leukemia 2001; 15: 688–689.
Garattini E, Parrella E, Diomede L, Gianni M, Kalac Y, Merlini L et al. ST1926, a novel and orally active retinoid-related molecule inducing apoptosis in myeloid leukemia cells: modulation of intracellular calcium homeostasis. Blood 2004; 103: 194–207.
Tanoue T, Maeda R, Adachi M, Nishida E . Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. EMBO J 2001; 20: 466–479.
Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE . Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. J Biol Chem 2008; 283: 19511–19520.
Parrella E, Gianni M, Cecconi V, Nigro E, Barzago MM, Rambaldi A et al. Phosphodiesterase IV inhibition by piclamilast potentiates the cytodifferentiating action of retinoids in myeloid leukemia cells. Cross-talk between the cAMP and the retinoic acid signaling pathways. J Biol Chem 2004; 279: 42026–42040.
Zhu J, Gianni M, Kopf E, Honore N, Chelbi-Alix M, Koken M et al. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RARalpha) and oncogenic RARalpha fusion proteins. Proc Natl Acad Sci USA 1999; 96: 14807–14812.
Hong SH, Privalsky ML . The SMRT corepressor is regulated by a MEK-1 kinase pathway: inhibition of corepressor function is associated with SMRT phosphorylation and nuclear export. Mol Cell Biol 2000; 20: 6612–6625.
Acknowledgements
We thank Rony Sager (Weizmann Institute, Rehovot), Elisabeth Goldsmith (UT South Western Medical Center, Dallas, TX, USA), Jiahuai Han (Scripps Research Institute, La Jolla, CA, USA), Hamin Zhou (Xiamen University, Xiamen, China), Stephen M Keyse and Robin Dickinson (Ninewells Hospital, Dundee, UK) for the reagents and useful discussion of the data. We are indebted to Roberto Piva (Center for Experimental Research and Medical Studies, Turin, Italy) for assistance with the procedures involving manipulation of the lentiviral vectors. The work of the PhD student, Maria Immacolata Loparco, is also acknowledged. This work was supported by grants from the Associazione Italiana per la Ricerca contro ilCancro (AIRC), the Fondazione Italo Monzino and the Negri-Weizmann Foundation were fundamental for the completion of this work. The work was also partially supported with grants from the Ministero della Salute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Gianni, M., Peviani, M., Bruck, N. et al. p38αMAPK interacts with and inhibits RARα: suppression of the kinase enhances the therapeutic activity of retinoids in acute myeloid leukemia cells. Leukemia 26, 1850–1861 (2012). https://doi.org/10.1038/leu.2012.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2012.50
Keywords
This article is cited by
-
Role of mitochondria and cardiolipins in growth inhibition of breast cancer cells by retinoic acid
Journal of Experimental & Clinical Cancer Research (2019)
-
S100A3 a partner protein regulating the stability/activity of RARα and PML-RARα in cellular models of breast/lung cancer and acute myeloid leukemia
Oncogene (2019)
-
Differentiation therapy revisited
Nature Reviews Cancer (2018)
-
The autophagy scaffold protein ALFY is critical for the granulocytic differentiation of AML cells
Scientific Reports (2017)
-
Catechins induced acute promyelocytic leukemia cell apoptosis and triggered PML-RARα oncoprotein degradation
Journal of Hematology & Oncology (2014)